Name: Pd___ Review for Ch 11 & 12 Test State the maximum

Name:______________________________ Pd___
Review for Ch 11 & 12 Test
State the maximum number of electrons allowed in each.
1. any p sublevel ______________
What is the maximum number of electrons that can be put into each of the following subshells?
2. 4d______________
3. 4f______________
4. 5f______________
5. 2s______________
____6. The alkali metals have how many valence electrons?
A. 8 B. 7 C. 2 D. 3 E. 1
____7. The halogens (Group 7) contain how many valence electrons?
A. 0 B. 1 C. 8 D. 7 E. None of the above
____8. 1s 2 2s 2 2p 6 3s 2 3p 2 is the correct electron configuration for which of the following atoms?
A. C B. Si C. Al D. S E. None of the above
____9. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 is the electron configuration for which of the following atoms?
A. Fe B. Cr C. Ar D. Ca E. None of the above
____10. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7 is the electron configuration for which of the following atoms?
A. Ca B. Ar C. Co D. Fe E. Cr
____11. A packet of energy of electromagnetic radiation is called
A. a photon B. a proton C. a wavelength D. a wave E. None of the above
____12. The maximum electron capacity of an f sublevel is
A. 6 B. 18 C. 14 D. 10 E. 2
____13. What element has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2 ?
A. Sn B. Ba C. Pb D. Po E. None of the above
____14. Order the elements S, Cl, and F in terms of increasing ionization energy.
A. F, S, Cl B. S, F, Cl C. S, Cl, F D. Cl, F, S E. F, Cl, S
____15. Order the elements S, Cl, and F in terms of increasing atomic radii.
A. F, S, Cl B. S, F, Cl C. Cl, F, S D. F, Cl, S
____16. Which has the larger atomic radius, S or Si?
____17. Which has the higher ionization energy, Rb or Cs?
E. S, Cl, F
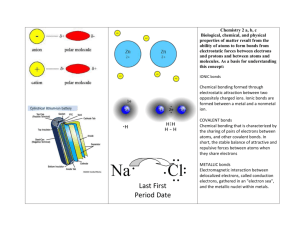
____18. Chemical bonds formed by the attraction of oppositely charged ions are called
A. ionic bonds B. coordinate bonds C. covalent bonds D. magnetic bonds
____19. Atoms with greatly differing electronegativity values are expected to form
E. None of the above
A. nonpolar covalent bonds B. ionic bonds C. no bonds D. triple bonds E. polar covalent bonds
____20. Metals typically have ______________ electronegativity values.
A. negative B. no C. high D. low E. two of these
____21. Which of the following has a double bond?
A. C
2
H
2
B. C
2
H
4
C. H
2
O D. CN -
____22. Which of the following has a triple bond?
A. H
2
S B. NH
3
C. H
2
O D. CO E. O
2
E. None of the above
____23. Which of the following has a double bond?
A. CO B. H
2
O C. O
2
D. H
2
S E. NH
3
24. Draw the Lewis electron structure for the Cl
2
molecule.
25. Draw the Lewis structure for the NH
4
+ ion.
26. Draw the Lewis structure for CCl
4
.
27. Draw the Lewis structure for N
2
.
28. Draw the Lewis electron structure for the sulfide ion.
____29. Which of the following has nonpolar bonds?
A. Br
2
B. H
2
S C. OF
2
D. HCl E. All are nonpolar.
____30. Which of these elements has the highest electronegativity?
A. Sr B. Y C. I D. Sb E. In
____31. Which of the following elements has the lowest electronegativity?
A. Ba B. Ca C. S D. H E. Cl
____32. Which of the following elements has the lowest electronegativity?
A. S B. Na C. Ca D. Cl E. Rb
____33. Which of the following contains only nonpolar bonds?
A. Mg
3
N
2
B. HCl C. H
2
O D. CH
4
E. Cl
2
____34. Which of the following has only nonpolar covalent bonds?
A. N
2
B. CO C. CCl
4
D. HI E. NaCl
____35. Which of the following is the most electronegative?
A. Cl B. Na C. Si D. Mg E. P
Answer Sheet
1. 6
2. 10
3. 14
4. 14
5. 2
6. E. 1
7. D. 7
8. B. Si
9. A. Fe
10. C. Co
11. A. a photon
12. C. 14
13. C. Pb
14. C. S, Cl, F
15. D. F, Cl, S
16. Si
17. Rb
18. A. ionic bonds
19. B. ionic bonds
20. D. low
21. B. C2H4
22. D. CO
23. C. O2
29. A. Br2
30. C. I
31. A. Ba
32. E. Rb
33. E. Cl2
34. A. N2
35. A. Cl
24. The Lewis electron structure for Cl2 is:
25. B. NH 4+ looks like:
26. The Lewis structure for CCl
4
is:
27. The Lewis structure for N
2
is:
28. The Lewis electron structure for sulfide is:
Standards Summary
NSES B.2.2: An element is composed of a single type of atom. When elements are listed in order according to the number of protons (called the atomic number), repeating patterns of physical and chemical properties identify families of elements with similar properties. This "Periodic Table" is a consequence of the repeating pattern of outermost electrons and their permitted energies.
CA 1.d: Students know how to use the periodic table to determine the number of electrons available for bonding.
NSES B.6.2: Electromagnetic waves result when a charged object is accelerated or decelerated. Electromagnetic waves include radio waves (the longest wavelength), microwaves, infrared radiation (radiant heat), visible light, ultraviolet radiation, x-rays, and gamma rays. The energy of electromagnetic waves is carried in packets whose magnitude is inversely proportional to the wavelength.
CA 1.c: Students know how to use the periodic table to identify alkali metals, alkaline earth metals and transition metals, trends in ionization energy, electronegativity, and the relative sizes of ions and atoms.
NSES B.2.1: Atoms interact with one another by transferring or sharing electrons that are furthest from the nucleus.
These outer electrons govern the chemical properties of the element.
NSES B.2.3: Bonds between atoms are created when electrons are paired up by being transferred or shared. A substance composed of a single kind of atom is called an element. The atoms may be bonded together into molecules or crystalline solids. A compound is formed when two or more kinds of atoms bind together chemically.
CA 2.a: Students know atoms combine to form molecules by sharing electrons to form covalent or metallic bonds or by exchanging electrons to form ionic bonds.
CA 2.e: Students know how to draw Lewis dot structures.
CA 2.b: Students know chemical bonds between atoms in molecules such as H_2 , CH_4 , NH_3 , H_2CCH_2, N_2, Cl_2 , and many large biological molecules are covalent.