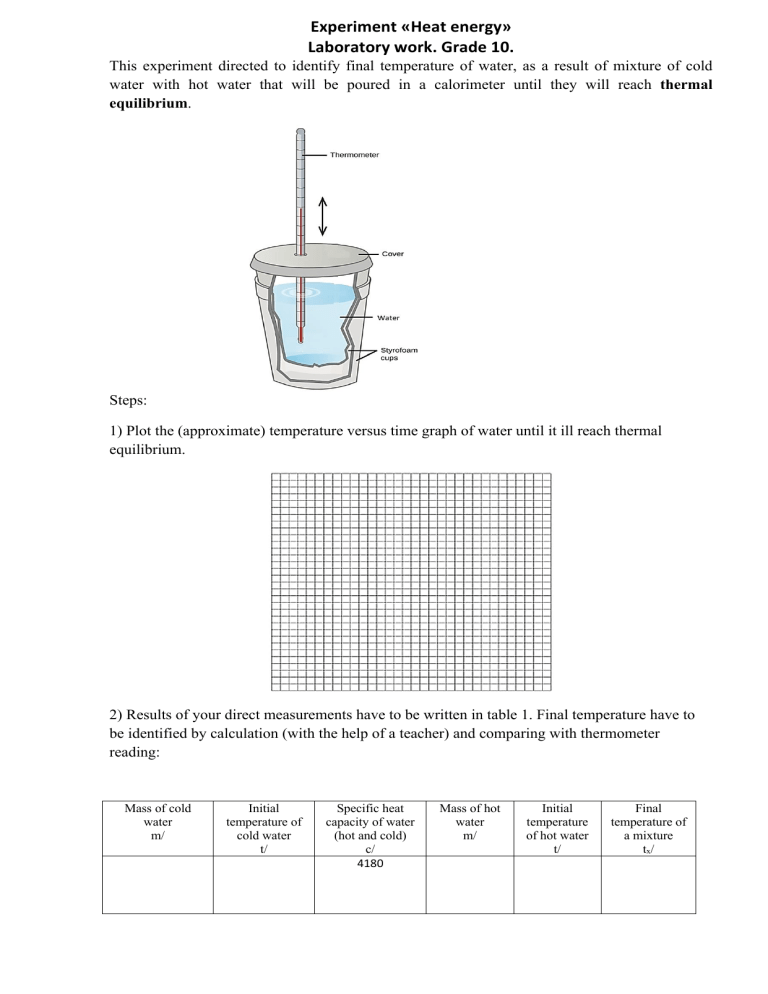
Experiment «Heat energy» Laboratory work. Grade 10. This experiment directed to identify final temperature of water, as a result of mixture of cold water with hot water that will be poured in a calorimeter until they will reach thermal equilibrium. Steps: 1) Plot the (approximate) temperature versus time graph of water until it ill reach thermal equilibrium. 2) Results of your direct measurements have to be written in table 1. Final temperature have to be identified by calculation (with the help of a teacher) and comparing with thermometer reading: Mass of cold water m/ Initial temperature of cold water t/ Specific heat capacity of water (hot and cold) c/ 4180 Mass of hot water m/ Initial temperature of hot water t/ Final temperature of a mixture tx/ Calculation of the final temperature: Explanation of the process: Improvements to the way we used to get results (in terms of method and instruments that we used during the experiment):