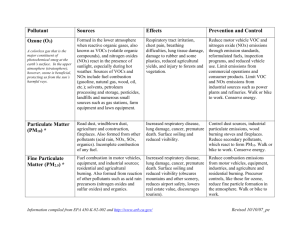
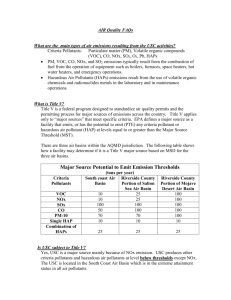
Air Resources Engineering (senior
advertisement

1) Which value is the largest? a. 1 ppb b. 1 ppm c. 0.01% 2) To convert from % to ppm, multiply by a. 10-6 b. 10-4 c. 10-2 d. 102 e. 104 3) Under standard conditions, 0.0245 is the number of a. Cubic meters taken up by 1 mole of gas b. Grams per liter of air c. Grams per mole of air d. Moles of gas in 1 cubic meter 4) How does the molecular weight of moist air higher compare to that of dry air? a. Lower b. Equal c. Higher 5) Air contains about 21% oxygen by a. Mass and volume b. Mass and moles c. Moles and volume 6) Which of the following is NOT a criteria pollutant? a. CO b. CO2 c. O3 d. NO2 e. PM2.5 7) Ozone is not directly emitted but forms in the atmosphere from reactions of a. Carbon dioxide and particulate matter b. Chlorofluorocarbons c. Nitrogen oxides and volatile organic compounds d. Particulate matter and volatile organic compounds 8) Nitrogen oxides (NOx) are the sum of a. N2 + NO + NO2 b. NO + NO2 c. NO + N2O d. NO2 + N2O 9) In PM2.5, the “2.5” refers to a. Particles larger than 2.5 µm b. Particles larger than 2.5 mm c. Particles less than 2.5 µg d. Particles smaller than 2.5 µm e. Particles smaller than 2.5 mm 10) Carbon monoxide originates mainly from a. Combustion of fuels b. Oxidation of carbon dioxide c. Secondary reactions d. Wind-blown sources 11) All of the following criteria pollutants cause respiratory irritation EXCEPT a. Carbon monoxide b. Ozone c. Nitrogen dioxide d. Particulate matter e. Sulfur dioxide 12) Which criteria pollutant has both primary and secondary sources a. Carbon monoxide b. Lead c. Ozone d. Particulate matter e. Sulfur dioxide 13) Average global temperatures have risen by approximately how much over the 20th century? a. 0 °C b. 1 °C c. 3 °C d. 5 °C 14) By how much do scientists estimate that global sea level rose in the 20th century? a. Zero b. 6-9 inches c. 3-4 feet d. 21-23 feet 15) Why is Earth warm enough to support life? a. Infrared radiation leaving Earth’s surface is absorbed by gases in the lower atmosphere. b. Radiation from the sun is absorbed by ozone in the stratosphere. c. X-rays and gamma rays excite gas molecules in the lower atmosphere. 16) Peak solar irradiance occurs in which wavelengths? a. Infrared b. Gamma c. Microwave d. Visible 17) Peak Earth irradiance occurs in which wavelengths? a. Infrared b. Gamma c. Microwave d. Visible 18) A greenhouse gas is one that a. Absorbs shortwave/visible radiation b. Absorbs longwave/infrared radiation c. Reflects shortwave/visible radiation d. Reflects longwave/infrared radiation 19) Which of the following is not a greenhouse gas? a. Carbon dioxide b. Chlorofluorocarbons c. Nitrogen d. Water vapor 20) Without the atmosphere’s natural greenhouse effect, Earth’s temperature would be a. 10-20 °C warmer b. 30-40 °C warmer c. 10-20 °C cooler d. 30-40 °C cooler 21) Vehicles are responsible for the majority of emissions of all of the following pollutants EXCEPT a. CO b. CO2 c. NOx d. VOC (volatile organic compounds) 22) In a four-stroke engine, pollutants are formed during which stroke? a. Intake b. Compression c. Power d. Exhaust 23) For stoichiometric combustion of gasoline, the air-fuel ratio on the basis of mass should be nearest a. 1 b. 1.5 c. 15 d. 50 24) An equivalence ratio of less than one means that combustion conditions are a. Catalytic b. Fuel lean c. Fuel rich d. Stoichiometric 25) Emissions of which two pollutants from gasoline-powered vehicles tend to parallel each other? a. CO and NOx b. CO and VOC c. NOx and VOC 26) Which vehicle-related pollutant is dominated by high-temperature equilibrium chemistry in terms of its formation? a. CO b. CO2 c. NOx d. VOC 27) Which vehicle-related pollutant is dominated by high-temperature equilibrium chemistry in terms of its formation? a. CO b. CO2 c. NOx d. VOC 28) Catalytic converters promote which of the following reactions (unbalanced)? a. CO O2 b. CO2 C c. NOx N2 d. PM O2 e. VOC O2 29) MTBE is used in which of the following applications? a. Catalytic converter b. Exhaust gas recirculation c. Hybrid powertrains d. Reformulated gasoline 30) Which of the following technologies has as its goal reduction of the combustion temperature? a. Catalytic converter b. Charcoal canister c. Exhaust gas recirculation d. Reformulated gasoline e. Vapor recovery 31) Selective catalytic reduction requires a a. Ethanol reservoir b. Filter c. Oxidation catalyst d. Urea tank 32) Selective catalytic reduction is used in a. Diesel-powered cars b. Gasoline-powered cars c. Both 33) Atmospheric pressure in Blacksburg is closest to a. 0.9 atm b. 1.0 atm c. 1.1 atm 34) The lapse rate describes how a. pressure changes with height b. temperature changes with height c. temperature changes with pressure d. the Coriolis force varies with latitude 35) Air pollution tends to be worst under which conditions? a. neutral b. stable c. unstable 36) The stability class depends on a. pressure and temperature b. pressure and wind c. radiation and temperature d. radiation and wind 37) The Gaussian plume equation is used to predict a. Concentrations in space b. Concentrations in time c. Emissions d. Violations of the NAAQS 38) Which of the following is NOT an assumption in the derivation of the Gaussian plume equation? a. Diffusion is unimportant in y- and z-directions b. Turbulence >> diffusion in the x-direction c. Wind and emissions are at steady state d. Wind is only in the x-direction 39) In the Gaussian plume equation, Q is the a. Emission rate b. Turbulent diffusivity c. Volume flow rate d. Wind velocity 40) If reflection is incorporated into the Gaussian plume equation, the calculated concentrations are a. double b. half c. higher by varying factors d. lower by varying factors e. unchanged 41) If you run an air pollution monitoring site for the Virginia Department of Environmental Quality, which rule most closely applies to your work? a. National Ambient Air Quality Standards b. State Implementation Plan c. New Source Performance Standards 42) If you consult for a civil engineering company that is building a new cement plant, which rule applies to your work? a. National Ambient Air Quality Standards b. State Implementation Plan c. New Source Performance Standards 43) Which of the following is NOT a unit of energy? a. British thermal unit (Btu) b. Joule (J) c. kilowatt hour (kWh) d. Newton second (Ns) 44) The heating value of coal refers to its a. maximum combustion temperature b. price for a Joule of energy c. energy content per mass d. mass per Btu of energy 45) The Class I designation refers to a a. level of air quality standard b. major point source of emissions c. nonattainment area d. National Park or Wilderness area 46) What is the major source of emissions of sulfur oxides? a. Power plants b. Diesel trucks c. Cement plants 47) Which of the following is not a low-sulfur fuel? a. Coal from Wyoming b. Gasified coal c. Petroleum oil d. Natural gas 48) What is the purpose of the liquid or solid added during flue-gas desulfurization? a. Absorption of the SO2 b. Catalysis of the SO2 c. Combustion of the SO2 d. Reaction with the SO2 49) In a counter-current flow scrubber, which two streams enter into or leave from the bottom? a. Liquid waste and clean exhaust b. Liquid waste and dirty exhaust c. Scrubbing solution and clean exhaust d. Scrubbing solution and dirty exhaust 50) What is the purpose of packing material in a scrubber? a. Absorb the gaseous pollutant b. Absorb the liquid waste c. Catalyze the reaction d. Enhance the surface area 51) Which nitrogen pollutant is most strongly associated with acid deposition? a. HNO3 b. NH3 c. N2O d. NO e. NO2 52) Which nitrogen pollutant is a greenhouse gas? a. HNO3 b. NH3 c. N2O d. NO e. NO2 53) Which nitrogen pollutant degrades visibility? a. HNO3 b. NH3 c. N2O d. NO e. NO2 54) What is the objective of flue/exhaust gas recirculation? a. Decrease equivalence ratio b. Increase equivalence ratio c. Reduce combustion temperature d. Reduce nitrogen in fuel 55) Which of the following pair is most similar for NOx control? a. Low-NOx burner and staged combustion b. Low-NOx burner and selective catalytic reduction c. Selective catalytic reduction and staged combustion 56) In flue-gas control of NOx, the desired product is a. NH3 b. N2O c. NO2 d. N2 57) Which NOx control method requires the addition of ammonia? a. Low-NOx burner b. Staged combustion c. Thermal de-NOx d. Three-way catalytic converter 58) VOCs are associated with all of the following EXCEPT a. corrosion b. ground-level ozone formation c. hazardous air pollutants (air toxics) d. secondary aerosol formation 59) Which of the following is not a major source of VOC emissions? a. chemical plants and refineries b. coal-fired power plants c. motor vehicles d. trees 60) An adsorption isotherm describes the a. equilibrium between gaseous and sorbed phases b. temperature at which a compound volatilizes c. vapor pressure as function of temperature 61) In an adsorption isotherm equation, X is typically the a. mass concentration of the gas in air b. mass of sorbent c. mass of sorbed compound per mass in air d. mass of sorbed compound per mass of sorbent 62) A charcoal strategy? a. b. c. canister for controlling evaporative emissions from your car relies on which VOC control adsorption condensation incineration 63) In the Southeastern US, the two major constituents of PM2.5 are a. crustal material and nitrate b. nitrate and sulfate c. elemental carbon and organic carbon d. organic carbon and sulfate 64) Secondary aerosol may be composed of any of the following EXCEPT a. ammonium b. elemental carbon c. nitrate d. organic carbon e. sulfate 65) For spherical particles larger than ~5 m, Stokes law describes the a. buoyancy force b. drag force c. slip correction d. terminal settling velocity 66) Brownian motion is due to a. the kinetic energy of particles b. the random motion of air molecules c. the thermal energy of particles d. the turbulent motion of air e. American Gladiators 67) Which of the following is NOT considered a particle collection mechanism in filters? a. diffusion b. gravitational settling c. impaction d. interception 68) Which two collection devices are best for ultrafine particles? a. cyclones and filters b. cyclones and settling chambers c. ESPs and filters d. ESPs and settling chambers 69) Ethanol has the following effects on vehicle emissions: a. decreased VOC and NOx b. decreased VOC and increased NOx c. increased VOC and decreased CO d. increased VOC and CO 70) Which of the following is not a biofuel? a. biodiesel b. cellulosic ethanol c. ethanol d. kerosene e. wood 71) The higher and lower heating values differ in that a. one is for hot days versus cold b. one is for melting versus vaporization c. water is emitted as a liquid versus a gas 72) In a fuel cell, the proton exchange membrane is where a. H+ ions pass from the anode to a cathode b. H+ ions recombine with O2 to form water c. H2 is separated into protons and electrons 73) Commercially available “flex fuel vehicles” can run on gasoline and a. biodiesel b. hydrogen c. ethanol d. vegetable oil 74) Current CO2 concentrations are higher than they have been over at least (select the largest known value) a. 150 years b. 2000 years c. 650,000 years d. 1.5 million years 75) The global warming potential combines a. CO2 equivalency and radiative forcing b. Lifetime and radiative forcing c. Radiative forcing and temperature 76) The 100-year GWP of a gas is always less than its 20-year GWP. a. True b. False c. Not enough information 77) Determination of R(t) in the GWP equation usually assumes a. Constant concentrations of a GHG b. First-order exponential decay of a GHG c. Linear decay of a GHG d. The answer varies by GHG 78) What fuel is the largest source of GHG emissions in the US? a. Coal b. Natural gas c. Petroleum 79) Emissions of a GHG in CO2-equivalents are calculated as the product of a. Emissions of the gas and its GWP b. Emissions of the gas and its lifetime c. Lifetime and GWP d. Lifetime and radiative forcing 80) The US is responsible for approximately what percentage of global GHG emissions? a. 10% b. 25% c. 33% d. 50% 81) Stabilizing CO2 emissions is equivalent to stabilizing its concentrations. a. True b. False c. Insufficient information 82) In the US, what is the biggest energy source for total consumption? a. Coal b. Natural gas c. Nuclear d. Oil 83) In the US, what is the biggest energy source for electricity generation? a. Coal b. Natural gas c. Nuclear d. Oil 84) Which country produces the most crude oil? a. Iran b. Russia c. Saudi Arabia d. United States e. Venezuela 85) In the drive for “clean” coal, which technology has the greatest potential for reducing CO2 emissions a. Coal washing b. Low-NOx burners c. Wet scrubbers d. Electrostatic precipitators 86) Syngas consists of a. CH4 and CO2 b. CO and H2 c. O2 and H2 d. O2 and steam 87) IGCC stands for a. Integrated Gasification Coal Cleaning b. Integrated Gasification Combined Cycle c. Intergovernmental Group on Climate Change 88) How does adding sulfate particles to the atmosphere cool the planet? a. Absorbs infrared radiation b. Destroys greenhouse gases c. Increases the albedo d. Reflects infrared radiation 89) A space-based sunshade would increase the Earth’s albedo. a. True b. False 90) Worsening of the ozone hole is associated with which geoengineering strategy? a. Iron fertilization b. Sea misting c. Sulfate aerosols d. Sunshade 91) Which of the following geoengineering technologies does NOT reduce sunlight reaching the surface? a. Iron fertilization b. Sea misting c. Sulfate aerosols d. Volcanic eruptions 92) The Chapman mechanism describes the balance of a. O, O2, O3 b. O, VOC, NOx c. O3, CFCs d. O3, OH, HO2 93) In atmospheric chemistry, a heterogeneous reaction occurs between a. a compound and M b. a compound and UV light c. a gas and a solid d. a radical and a non-radical 94) Which reaction absorbs most UVB radiation? a. O2 + h 2 O b. O + O2 O3 c. O3 + h O2 + O d. O + O3 2 O2 95) Which of the following is directly responsible for the catalytic destruction of ozone? a. CCl3F b. Cl c. Cl2 d. ClONO2 96) Which of the following is an inactive form of chlorine? a. CCl3F b. Cl c. Cl2 d. ClONO2 97) Which of the following is a form of active chlorine? a. CCl3F b. Cl c. Cl2 d. ClONO2 98) The severity of the Antarctic ozone hole is worst during the Southern Hemisphere a. winter b. spring c. summer d. fall