Written Information on Chemistry Summative - SCH4U1-02-2010
advertisement

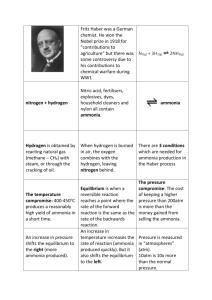
Ashkon Poruheidary Chemistry Summative Project What is Ammonia? Ammonia is a molecule consisting of Nitrogen and Hydrogen, covalently bonded with and bond angular of 107, with formula of NH3. This colourless has contributes significantly to the nutritional and pharmaceutical needs of people around the world. Have you ever asked yourself that with 6.92 billion people living on earth, how does the food production industry is able to feed all of the people? Ways of Production: Two main types of production process for ammonia synthesis gas are currently in operation in Europe: Steam reforming of natural gas or other light hydrocarbons (Natural Gas Liquids, Liquefied Petroleum Gas, Naphtha) Partial oxidation of heavy fuel oil or vacuum residue About 85% of world ammonia production is based on steam reforming process. Steam reforming of natural gas: 1. The first step in the process is to remove sulphur compounds from the feedstock because sulphur deactivates the catalysts used in subsequent steps. Sulphur removal requires catalytic hydrogenation to convert sulphur compounds in the feedstocks to gaseous hydrogen sulphide: H2 + RSH → RH + H2S (gas) 2. The gaseous hydrogen sulphide is then absorbed and removed by passing it through beds of zinc oxide where it is converted to solid zinc sulphide: H2S + ZnO → ZnS + H2O 3. Catalytic steam reforming of the sulphur-free feedstock is then used to form hydrogen plus carbon monoxide: CH4 + H2O → CO + 3H2 4. The next step then uses catalytic shift conversion to convert the carbon monoxide to carbon dioxide and more hydrogen: CO + H2O → CO2 + H2 5. The carbon dioxide is then removed either by absorption in aqueous ethanolamine solutions or by adsorption in pressure swing absorbers (PSA) using proprietary solid adsorption media. 6. The final step in producing the hydrogen is to use catalytic methanation to remove any small residual amounts of carbon monoxide or carbon dioxide from the hydrogen: CO + 3H2 → CH4 + H2O CO2 + 4H2 → CH4 +2H2O 7. To produce the desired end-product ammonia, the hydrogen is then catalytically reacted with nitrogen (derived from process air) to form anhydrous liquid ammonia. This step is known as the ammonia synthesis loop (also referred to as the Haber-Bosch process): 3H2 + N2 → 2NH3 1 Ashkon Poruheidary The Connections to Chemistry 12 Course: 1. The role of Catalysts The catalyst has no effect on the position of chemical equilibrium; rather, it provides an alternative pathway with lower activation energy and hence increases the reaction rate, while remaining chemically unchanged at the end of the reaction. The first Haber–Bosch reaction chambers used osmium and ruthenium as catalysts. However, under Bosch's direction in 1909, a much less expensive iron-based catalyst was discovered that is still used today. Part of the industrial production now takes place with a ruthenium rather than an iron catalyst because this more active catalyst allows reduced operating pressures. 2. Le chatelier’s Principle: The ammonia production reaction is an exothermic reaction, thus heat releases as the reaction proceeds. Le chatelier’s Principle predicts that the yield of ammonia is greater at lower temperature. This way there is more ammonia produced. However, this would make another problem; low temperature would decrease the rate of the reaction. This is crucial when we examine the profits of the company producing ammonia. Le chalier’s principle also predicts that the yield of ammonia is greater at higher pressures. High pressure platns are expensive to build and maintain, however.. Modern plants operate at pressures in the range of 20,000 kPa to 30,000 Kpa. 3. Effects of Pressure on Rate of Reaction the effects of pressure on rate of reaction comes into the action, at high pressure, the rate of reaction will increase according to the collision theory, the high pressure would cause more successful collisions, therefore the rate of reaction would be increase. 2 Ashkon Poruheidary 3