Document Template
advertisement

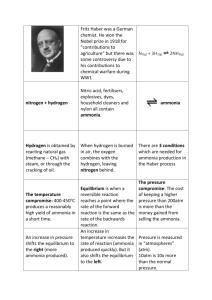
SEMI Publication Improvement Proposal Form Submitted By: Mark Ripkowski Company: CONSCI Address: Date:__11/02/2015_____________________________ Document Number: _R5671C____________________ Document Title: Revision to SEMI C3.12-1109, Specification for Ammonia (NH3) in Cylinders, 99.998% Quality Telephone: Email: _mark@conscicorp.com___________________ Details Location of Change: Page(s) 4,6___________ Section(s) 8.3, 8.3.3.2, 8.3.3.3 Figure(s) Table(s) Note(s) Other _____________ Are you a Standards Program Member? Yes Χ No Proposed Change and Reasons From: 8.3 Water — The method for the determination of water in ammonia is based on its decomposition by the reaction 2NH3 → N2 + 3H2, which occurs completely and irreversibly when ammonia is passed over a nickel catalyst at 1000°C. Water passes through the catalyst unchanged. Therefore, the water content of ammonia may be determined by measuring the dew point of the stream of hydrogen and nitrogen produced by decomposition of ammonia. 8.3.3.2 Subtract twice the concentration of oxygen (as measured by the method specified in ¶ 8.3) in the sample from the concentration of water calculated in ¶ 8.3.3.1. This is necessary because oxygen in the ammonia reacts with the hydrogen formed by decomposition to produce additional water 8.3.3.2 FTIR (Foutier Transform Infrared) or Cavity Ring Down Spectrometer configured to meet the specification may be used. To: 8.3 Water — The method for the determination of water in ammonia is based on its decomposition by the reaction 2NH3 → N2 + 3H2, which occurs completely and irreversibly when ammonia is passed over a nickel catalyst at 1000°C. Water passes through the catalyst unchanged. Therefore, the water content of ammonia may be determined by measuring the dew point of the stream of hydrogen and nitrogen produced by decomposition of ammonia. FTIR (Fourier Transform Infrared) or Cavity Ring Down Spectrometer configured to meet the specification may be used. 8.3.3.2 Subtract twice the concentration of oxygen (as measured by the method specified in ¶ 8.13) in the sample from the concentration of water calculated in ¶ 8.3.3.1. This is necessary because oxygen in the ammonia reacts with the hydrogen formed by decomposition to produce additional water 8.3.3.3 FTIR (Foutier Transform Infrared) or Cavity Ring Down Spectrometer configured to meet the specification may be used. Justification: Editorial in nature. The oxygen concentration is determined in Section 8.1. Paragraph 8.3 consists, in its entirety, of “Water – The method… decomposition of ammonia”. In the structure in the ballot, this paragraph allows using the alternative methods for reporting, but not for measurement. It seems unlikely this was the intent. *Corrections to obvious formatting or typographical errors (e.g., misspellings) can be fixed as editorial changes by Publication Improvement Proposal (PIP) form submission. Changes that do not qualify as editorial (refer to the SEMI Standards Procedure Guide) will need to follow normal Letter Ballot procedures. Approved by: Standards Staff Laura Nguyen Date: 11/03/2015 Committee Chair Mohamed Saleem Date: 11/03/2015 Committee Chair Steve Lewis Date: 11/03/2015 Please return this form to SEMI, Standards Publications, 3081 Zanker Road, San Jose, CA 95134; standardspublishing@semi.org SEMI X##-#### © SEMI 2001 2