biochem ch 31 [3-6
advertisement

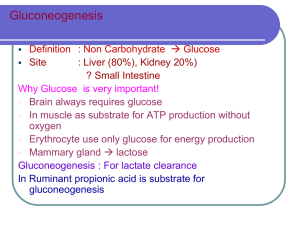
BioChem Ch 31 Glucose Metabolism in Liver Glycolysis converting glucose to pyruvate provides carbon for synthesis of fatty acids; glycerol 3-phosphate from glycolytic intermediates combines with fatty acids to form triacylglycerols, which are secreted into blood in VLDL Measurement of blood and urine ketones indicate level of starvation or presence of diabetic ketoacidosis (DKA) o Urine reagent strips don’t detect major blood ketone β-hydroxybutyrate o Cyclic enzymatic method removes acetoacetate and makes color change with β-hydroxybutyrate Gluconeogenesis Gluconeogenesis – process by which glucose is synthesized from noncarbohydrate precursors; occurs mainly in liver under fasting conditions o Under most extreme conditions of starvation, kidney cortex may produce glucose; most of this used by kidney cortex, but some may enter bloodstream Starting with pyruvate, mostly reversals of glycolysis; only differ at three points (enzymes regulated to determine whether glycolysis or gluconeogenesis predominates) Kussmaul respirations in DKA patient due to acidosis-induced stimulation of respiratory center in brain o Severe hyperglycemia causes osmotic diuresis, which causes lower blood volume o Volume depletion may be aggravated by vomiting o Causes dehydration (dry mucous membranes, loss of skin turgor), low BP, and rapid heartbeat o Hypoglycemic coma has flushed, wet skin, not dry skin seen in DKA Major carbon sources for gluconeogenesis are lactate, glycerol, and amino acids (particularly alanine) o Lactate produced by adipocytes during fed state o Glycerol released from adipose stores of triacylglycerol o Alanine produced in muscle from other amino acids and from glucose o Ethanol metabolism gives rise to only acetyl-CoA, so carbons can’t be used for gluconeogenesis Lactate dehydrogenase oxidizes lactate to pyruvate, generating NADH Alanine aminotransferase converts alanine to pyruvate Impaired fasting glucose (IFG) – prediabetes Carbons of glycerol gluconeogeneic because they form dihydroxyacetone phosphate (DHAP; glycolytic intermediate) Glucocorticoids (natural cortisol or drug prednisone or dexamethasone) produced in adrenal cortex in response to stress; stimulate degradation of muscle protein, allowing increased amounts of amino acids to be substrates for gluconeogenesis Fatty acids with odd number of carbon atoms (obtained from vegetables) relatively minor precursors of glucose β-oxidation of fatty acids produces acetyl-CoA; because pyruvate dehydrogenase reaction irreversible, acetylCoA doesn’t form pyruvate for gluconeogenesis, so acetyl-CoA enters TCA cycle and is converted to malate o For every 2 carbons of acetyl-CoA converted to malate, 2 carbons released as CO2, so no net synthesis of glucose from acetyl-CoA In glycolysis, PEP converted to pyruvate by pyruvate kinase; in gluconeogenesis, series of steps needed to reverse this o Pyruvate carboxylated by pyruvate carboxylase to form oxaloacetate (requires biotin); also anaplerotic reaction of TCA cycle (takes place in mitochondria) o CO2 added to pyruvate to form oxaloacetate released in reaction catalyzed by PEPCK, which makes PEP (takes place equally in either mitochondria or cytosol) Oxaloacetate, generated from pyruvate or amino acids, doesn’t cross mitochondrial membrane o Either decarboxylated to form PEP by mitochondrial PEPCK or converted to malate or aspartate o Conversion of oxaloacetate to malate requires NADH o PEP, malate, and aspartate can be transported into cytosol o After malate or aspartate enters cytosol, it’s converted back to oxaloacetate by reversing reaction o Conversion of malate to oxaloacetate generates NADH o Whether oxaloacetate transported across mitochondrial membrane as malate or aspartate depends on need for reducing equivalents in cytosol o Oxaloacetate produced from malate or aspartate in cytosol converted to PEP by cytosolic PEPCK Excessive ethanol metabolism blocks production of gluconeogenic precursors; ethanol rapidly oxidized in liver, so NADH/NAD+ ratio much higher than normal fasting liver o High NADH levels drive lactate dehydrogenase reaction toward lactate, so lactate can’t enter gluconeogenesis pathway, and pyruvate generated from alanine converted to lactate o Because glycerol oxidized by NAD+ during conversion of DHAP, conversion of glycerol to glucose inhibited when NADH levels elevated o Normally, amino acids that form intermediates of TCA cycle converted to malate, which enters cytosol and is converted to oxaloacetate; elevated NADH levels inhibit conversion of malate to oxaloacetate o Too much alcohol = no gluconeogenesis, and alcoholic becomes hypoglycemic Remaining steps of gluconeogenesis after PEP made occur in cytosol o For every 2 molecules of glyceraldehyde 3-phosphate formed, one converted to DHAP and both products condense to form F1,6P2 o Because glycerol forms DHAP, it enters gluconeogenesis here Fructose 1,6-bisphosphatase releases Pi from F1,6P2 to form fructose 6-phosphate (ATP not produced because it’s a low-energy phosphate bond) Glucose 6-phosphatase hydrolyzes Pi from glucose 6-phosphate, and free glucose released into blood o Phosphate bond is low-energy, so no ATP formed, so NOT reversal of glycolysis o Glucose 6-phosphatase located in membrane of ER and is also used to produce blood glucose from breakdown of liver glycogen Gluconeogenesis stimulated during fasting, prolonged exercise, high-protein diet, and under stress o Glycerol released from adipose tissue whenever levels of insulin low and levels of glucagon or stress hormones (epinephrine and cortisol) elevated in blood o Amino acids released from muscle whenever insulin low or when cortisol elevated o Amino acids also available for gluconeogenesis when dietary intake of protein high and intake of carbohydrate low Regulated steps of gluconeogenesis are pyruvate PEP, F1,6P2 fructose 6-phosphate, and glucose 6phosphate glucose o Steps correspond to those in glycolysis catalyzed by regulatory enzymes o Enzymes involved in these steps of gluconeogenesis different from reverse reactions of glycolysis o Net flow of carbon (gluconeogenesis or glycolysis) depends on activity or amount of these enzymes Pyruvate for gluconeogenesis derived from lactate and amino acids (particularly alanine); not converted to acetyl-CoA under conditions that favor gluconeogenesis because pyruvate dehydrogenase relatively inactive o Pyruvate converted to oxaloacetate by pyruvate carboxylase, then converted to PEP by PEPCK Under conditions of fasting, insulin levels low, and glucagon levels elevated; fatty acids and glycerol released from triacylglycerol stores of adipose tissue o Fatty acids travel to liver where they undergo β-oxidation producing acetyl-CoA, NADH, and ATP o Concentration of ADP decreases, resulting in phosphorylation of pyruvate dehydrogenase to inactive form, so pyruvate can’t be converted to acetyl-CoA Acetyl-CoA produced by oxidation of fatty acids activates pyruvate carboxylase; pyruvate derived from lactate or alanine converted to oxaloacetate o Acetyl-CoA levels reciprocally regulate pyruvate dehydrogenase and pyruvate carboxylase (high levels of acetyl-CoA inhibit pyruvate dehydrogenase and activate pyruvate carboxylase) Oxaloacetate produces PEP (catalyzed by PEPCK); cytosolic PEPCK is inducible enzyme; major inducer (cAMP) increased by glucagon during fasting and by epinephrine during exercise or stress o cAMP activates PKA, which phosphorylates CREB that stimulates transcription of PEPCK gene o Cortisol also induces PEPCK but through different regulator site on PEPCK promoter When glucagon elevated, liver pyruvate kinase phosphorylated (inactivated) by mechanism that involves cAMP and PKA, so PEP not reconverted to pyruvate (continues down gluconeogenesis) Glucocorticoids – steroid hormones that stimulate gluconeogenesis (induce synthesis of PEPCK) Futile substrate cycle prevented at conversion of F1,6P2 to fructose 6-phosphate because under conditions that favor gluconeogenesis, concentrations of compounds that activate glycolytic PFK-1 low o F2,6P2 (levels regulated by insulin and glucagon) and AMP allosterically inhibit fructose 1,6bisphosphatase o Synthesis of fructose 1,6-bisphosphatase induced during fasting Glucose 6-phosphatase catalyzes conversion of glucose 6-phosphate to glucose, released from liver cell o Glucokinase (catalyzes reverse reaction in glycolysis) relatively inactive during gluconeogenesis; not active during fasting because blood glucose lever lower than S0.5 of enzyme o Glucokinase is inducible during fed state (when blood glucose and insulin levels elevated) and decreased in fasting state During gluconeogenic reactions, 6 ATP cleaved; 2 pyruvate required for synthesis of 1 glucose o As 2 pyruvate carboxylated by pyruvate carboxylase, 2 ATP hydrolyzed o PEPCK requires 2 GTP to convert 2 oxaloacetate to 2 PEP o 2 ATP used when two 3-phosphoglycerate phosphorylated to two 1,3-bisphosphoglycerate o NADH required for conversion of 1,3-bisphosphoglycerate to glyceraldehyde 3-phosphate o Under fasting conditions, energy required for gluconeogenesis obtained from β-oxidation of fatty acids o Defects in fatty acid oxidation can lead to hypoglycemia because of reduced fatty acid-derived energy production within liver Glucose 6-phosphatase used in both glycogenolysis and gluconeogenesis to produce free glucose from glucose 6-phosphate Changes in Blood Glucose Levels After a Meal Blood glucose rises 30-60 minutes after high-carb meal, then decreases to be fasting levels 2 hours after meal o Blood glucose levels decline because cells take up and metabolize glucose If blood glucose continued to rise after meal, high concentration of glucose cause release of water from tissues, resulting in tissue dehydration and impaired function o If hyperglycemia becomes severe, hyperosmolar coma could result from brain dehydration If blood glucose levels continue to drop, tissues that depend on glucose suffer from lack of energy; brain can’t produce adequate amount of ATP; light-headedness and dizziness result, followed by drowsiness, then coma o RBCs don’t produce enough ATP to maintain integrity of membranes, and hemolysis decreases transport of oxygen to tissues of body o Eventually all tissues that rely on oxygen for energy production fail, and person dies As concentration of blood glucose approaches normal fasting range 2 hours after meal, glycogenolysis activated in liver; liver glycogen is primary source of blood glucose during first few hours of fasting Carbon for gluconeogenesis (performed by liver) supplied by other tissues o Exercising muscle and RBCs provide lactate through glycolysis o Muscle provides amino acids by degradation of protein o Glycerol released from adipose tissue as triacylglycerol stores metabolized Even after prolonged fast, blood glucose levels don’t decrease dramatically Diabetics that inject too much insulin experience hypoglycemia-induced hyperactivity of SNS, followed by neuroglycopenia (inadequate glucose supply to brain, causing confusion, speech disturbances, possible seizures) o Excess insulin inhibits lipolysis and ketone body synthesis, so alternative fuels not available Anorexics have very slow decrease in blood glucose because of hepatic gluconeogenesis and adipose lipolysis, which produces fatty acids that are used as fuel and converted to ketone bodies by liver o Oxidation of fatty acids and ketone bodies by brain and muscle reduce need for blood glucose Arginine and leucine stimulate insulin release from pancreas (as well as glucose does) Any excess glucose converted to storage fuels: glycogen synthesized and stored in liver, glucose converted to fatty acids and glycerol moiety that reacts with fatty acids to produce triacylglycerols o Triacylglycerols formed from glucose packaged in VLDL and transported to adipose tissue, where fatty acids stored in adipose triacylglycerols; VLDL can deliver triglycerides (fatty acids) to muscle for immediate oxidation if required As concentration of glucose increases in portal vein, velocity of glucokinase reaction increases (also induced in response to high carb diet or elevated insulin levels) Insulin promotes storage of glucose as glycogen by countering effects of glucagon-stimulated phosphorylation o Response to insulin activates phosphatases that dephosphorylate glycogen synthase (leads to glycogen synthase activation) and glycogen phosphorylase (inhibited by dephosphorylation) Insulin promotes synthesis of triacylglycerols released from liver into blood as VLDL All tissues require glucose for pentose phosphate pathway; many tissues use glucose for synthesis of glycoproteins and other carbohydrate-containing compounds Insulin stimulates transport of glucose into adipose and muscle cells by promoting recruitment of glucose transporters to PM; other tissues have different types of transporters and aren’t affected by insulin Muscle also synthesizes glycogen after meal, stimulated by insulin o Insulin greatly stimulates transport of glucose into muscles but slightly stimulates transport into liver Glucagon receptors activate adenylate cyclase, increasing cAMP, activating PKA, which phosphorylates (inactivates) glycogen synthase; PKA stimulates glycogen degradation by activating phosphorylase kinase, which phosphorylates (activates) glycogen phosphorylase, which catalyzes phosphorolysis of glycogen (first step) 2 hours after meal, glycogenolysis starts; 4 hours after meal, gluconeogenesis starts Regulatory mechanisms to prevent glycolysis while gluconeogenesis going on inactivate enzymes pyruvate kinase, PFK-1, and glucokinase during fasting o Pyruvate (derived from lactate and alanine) converted by gluconeogenic pathway to PEP; reverse reaction inhibited because glucagon-stimulated phosphorylation inactivates pyruvate kinase o Low F2,6P2 allows F-1,6-BPase to function more (converting F1,6P2 to F6P; high F2,6P2 stimulates PFK-1 Low F2,6P2 caused by phosphorylation of PFK-2 by PKA (activated in response to glucagon) o Because glucokinase has high S0.5 (Km) for glucose, glucose concentrations low in liver during fasting, so glucose released into blood o Gluconeogenic enzymes active in fasting conditions; pyruvate carboxylase activated by acetyl-CoA (derived from oxidation of fatty acids); PEPCK, F1,6BPase, and G6Pase induced Hormonal changes during fasting stimulate breakdown of adipose triacylglycerols, releasing fatty acids and glycerol; glycerol serves as source of carbon for gluconeogenesis; fatty acids become major fuel of body o Fatty acids oxidized to acetyl-CoA in liver to contribute to gluconeogenesis o In prolonged fast, acetyl-CoA converted to ketone bodies, which serve as fuel for muscle and brain Those with diabetes have excessive hepatic gluconeogenesis even though blood glucose levels elevated During prolonged fasting, tissues use predominantly fuels derived from adipose triacylglycerols (fatty acids and ketone bodies); dramatic elevation of blood ketone bodies after 3-5 days of fasting o As result of reduced glucose use, rate of gluconeogenesis in liver decreases as does production of urea Amino acids from protein degradation major gluconeogenic precursors, so reducing glucose requirements reduces rate of protein degradation and rate of urea formation o Starving person usually dies of severe protein loss that causes malfunction of major organs During 12-hour fast, glycogenolysis is major source of blood glucose; by 16 hours, glycogenolysis and gluconeogenesis contribute equally o After 30 hours, liver glycogen stores depleted, so gluconeogenesis is primary source of glucose During exercise, liver maintains blood glucose levels through glucagon and epinephrine-induced glycogenolysis and gluconeogenesis o Muscle cells lack glucose-6-phosphatase, so glucose can’t be produced for export from muscle Glucose Production Gluconeogenesis doesn’t make new glucose; it’s recycling products made from glucose back to glucose o New glucose obtained from plants and other foods we eat Cori cycle – pyruvate reduced to lactate by working muscles or tissues, released into bloodstream, and reconverted to glucose by gluconeogenesis in liver Pyruvate transaminated to alanine; alanine from muscle recycled to glucose in liver (glucose-alanine cycle) Glucose can be used to produce nonessential amino acids, which can later be converted back to glucose All amino acids (essential included) converted to glucose in humans originally synthesized from glucose Glycerol derived from glucose via DHAP intermediate of glycolysis; fatty acids esterified to glycerol and stored as triacylglycerol; when fatty acids released, glycerol travels to liver and is reconverted to glucose