Name Date ______ Block _____ 1⁄2 Life and Radioactive Decay For
Name _______________________ Date ____________ Block _____
1⁄2 Life and Radioactive Decay
For more practice on section 7.2 see pg 357 # 3-6, 8-10, 12-18
1.
The half-life of radon-222 is 4 days. Using this example, explain what 1⁄2 life is.
2.
If you started with 100 million radioactive atoms, how many would you have left after: One 1⁄2 life ?
Two 1⁄2 lives? Three half-lives? a.
What happens to the radioactive atoms once they decay?
3.
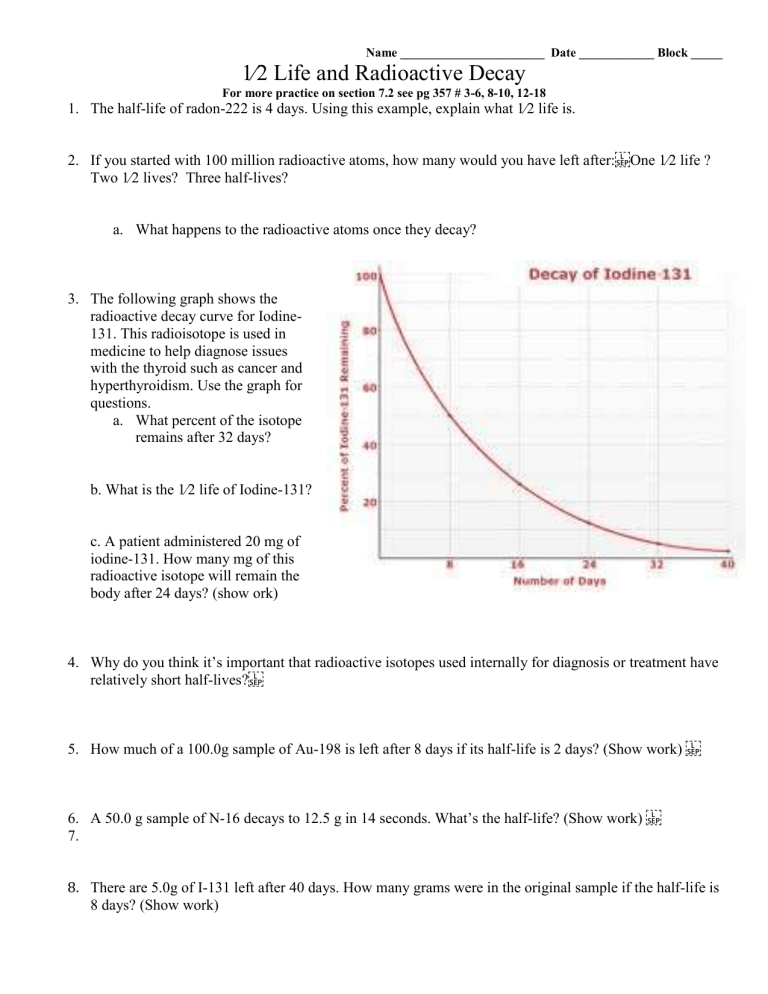
The following graph shows the radioactive decay curve for Iodine-
131. This radioisotope is used in medicine to help diagnose issues with the thyroid such as cancer and hyperthyroidism. Use the graph for questions. a.
What percent of the isotope remains after 32 days? b. What is the 1⁄2 life of Iodine-131? c. A patient administered 20 mg of iodine-131. How many mg of this radioactive isotope will remain the body after 24 days? (show ork)
4.
Why do you think it’s important that radioactive isotopes used internally for diagnosis or treatment have relatively short half-lives?
5.
How much of a 100.0g sample of Au-198 is left after 8 days if its half-life is 2 days? (Show work)
6.
A 50.0 g sample of N-16 decays to 12.5 g in 14 seconds. What’s the half-life? (Show work)
7.
8.
There are 5.0g of I-131 left after 40 days. How many grams were in the original sample if the half-life is
8 days? (Show work)
1⁄2 Life and Radioactive Decay
1.
The half-life of radon-222 is 4 days. Using this example, explain what 1⁄2 life is.
After 4 days, half of the sample of radon-222 will have decayed into a stable isotope.
2.
If you started with 100 million radioactive atoms, how many would you have left after: One 1⁄2 life ?
50 million atoms
Two 1⁄2 lives?
25 million atoms Three half-lives? 12.5 million atoms a.
What happens to the radioactive atoms once they decay?
3.
The following graph shows the radioactive decay curve for Iodine-131. This radioisotope is used in medicine to help diagnose issues with the thyroid such as cancer and hyperthyroidism. Use the graph for questions. a.
What percent of the isotope remains after 32 days?
6.25 % b. What is the 1⁄2 life of Iodine-131?
8 days c. A patient administered 20 mg of iodine-131. How many mg of this radioactive isotope will remain the body after 24 days? (show work)
X = 2.5 mg
4.
Why do you think it’s important that radioactive isotopes used internally for diagnosis or treatment have relatively short half-lives?
So that the radioactive material will not stay in the patient’s system for long periods of time.
5.
How much of a 100.0g sample of Au-198 is left after 8 days if its half-life is 2 days? (Show work)
After 2 days, there will be 50.00 g. After 4 days, there will be 25.00 g. After 6 days, there will be
12.50 g. After 8 days, there will be 6.250 g.
6.
A 50.0 g sample of N-16 decays to 12.5 g in 14 seconds. What’s the half-life? (Show work) 12.5 g is
1⁄4 of 50.0 g. This means that the sample has gone through 2 half-lives and the half-life of N-16 is 7
seconds.
7.
There are 5.0g of I-131 left after 40 days. How many grams were in the original sample if the half-life is
8 days? (Show work)
After 32 days there was 10. g. After 24 days, there was 20. g. After 16 days, there was 40. g. After 8 days, there was 80. g. So, the original sample was 160 g.