DR.Mojibian
1390
The Ob-Gyn Clerkship: Your Guide to Success
Tools for the Clerkship, contained in this document:
1.
2.
3.
4.
5.
6.
7.
8.
Sample obstetrics admission note
Sample delivery note
Sample operative note
Sample postpartum note
a. Vaginal delivery
b. Cesarean section orders/note
Sample gynecologic history & physical (H&P)
Admission orders
Commonly-used abbreviations
Spanish lesson
1
DR.Mojibian
1.
1390
Sample Admission to Labor and Delivery Note
Date & time
Identification (includes age, gravidity, parity, estimated gestational age, and reason for admission):
26yo G3P1A1 @ 38W5D EGA presents with painful contractions since noon. Pt reports good fetal movement,
and denies rupture of membranes or vaginal bleeding.
LMP:
Estimated date of confinement (EDC):
Chief complaint:
History of present illness (includes Prenatal Care (PNC): Labs, including HIV, GBS, GDM/HTN, # PNC
visits, wt gain, s=d, etc.
Past history:
Obstetrics:
List each pregnancy (NSVD, wt 4000 grams, complicated by gestational diabetes and
shoulder dystocia)
Gynecology:
PMH and PSH:
Medications: PNV, FeSO4
Allergies: No Known Drug Allergies (NKDA)
Social history: Ask about Tobacco/EtOH/Drugs
Physical exam (focused):
General and Vital signs
Lungs
CV – (Many pregnant women have a grade 1-2/6 systolic ejection murmur
Abd – Gravid, fundus non-tender (NT), fundal height (FH) 38cm, Leopold maneuvers:
Fetus is vertex (VTX), estimated fetal weight (EFW) 3300 gm
Sterile speculum examination if indicated to rule out spontaneous rupture of membranes
(SROM)
Sterile vaginal exam (SVE) = 4cm/80%/VTX/ –1 as per Dr. Smith/time
Ext – No Cyanosis, clubbing or edema (C/C/E), NT
Pertinent Labs:
Ultrasound: Date: 10 wks by crown-rump length (CRL)
Date: 20 wks, no anomalies
Assessment: 26yo G3P1 at term, in labor fetal heart rate tracing (FHRT) reassuring
Intrauterine pregnancy (IUP) at 39 weeks gestation
FHRT – Baseline 140’s, accelerations present, no decelerations
Contractions – q 4-5 min
Any pertinent past medical or surgical history
Plan: Admit to L&D
NPO except ice chips
IV – D5LR at 125 cc/hr
Continuous electronic fetal monitoring
CBC, T&S, RPR
Anticipate NSVD
2
DR.Mojibian
2.
1390
Sample Delivery Note
Date and time:
Summary: NSVD of a live male, 3000 gm and Apgars 9/9. Delivered LOA, no nuchal cord, light meconium.
Nose and mouth bulb suctioned at perineum; body delivered without difficulty. Cord clamped and cut. Baby
handed to nurse. Placenta delivered spontaneously, intact. Fundus firm, minimal bleeding. Placenta appears
intact with 3 vessel cord. Perineum and vagina inspected – small 2nd degree perineal laceration repaired
under local anesthesia with 2-0 and 3-0 chromic suture in the usual fashion. EBL 350cc. Hemostasis. Pt
tolerated procedure well, recovering in LDR. Infant to WBN.
3.
Sample Operation Note
Date and Time:
Pre-op Diagnosis: Symptomatic uterine fibroids or Pregnancy at term, failure to progress`
Postop Diagnosis: Same
Procedure: TAH/BSO or Cesarean Section
Surgeon (Attending):
Residents:
Anesthesia: GET (general endotracheal, others include spinal, LMA, IV sedation)
Complications: None
EBL: 300 cc
Urine Output: 200 cc, clear at the end of procedure
Fluids: 2,500 cc crystalloid (include blood or blood products here)
Findings: Exam under anesthesia (EUA) and operative
Spécimen: Cervix/uterus
Drains: If placed
Disposition: Recovery room, Surgical ICU, etc
4a.
Sample Postpartum Notes (Soap format)
Date and Time:
Subjective: Ask every patient about:
• Breastfeeding – are they breastfeeding/planning to? How is it going? Baby able to latch on?
• Contraceptive plan with relevant sexual history
• Lochia (vaginal bleeding) – Clots? How many pads?
• Pain – cramps/perineal pain/leg pain? Relief with medication? Do they need more pain meds?
Objective:
• Vital signs and note tachycardia, elevated or low BP, maximum and current temperature
• Focused physical exam including
o Heart
o Lungs
o Breasts: engorged? Nipples – skin intact?
o Abd: Soft? Location of the uterine fundus – below umbilicus? Firm? Tender?
o Perineum: Assess lochia (blood on pad, how old is pad?)
Visually inspect perineum – Hematoma? Edema? Sutures intact?
o Extremities: Edema? Cords? Tender?
• Postpartum labs: Hemoglobin or hematocrit
3
DR.Mojibian
1390
Assessment/Plan: PPD#_ S/P NSVD or Vacuum or Forceps (with 4th-degree laceration, with pre-eclampsia
s/p Magnesium Sulfate)
• General assessment – Afebrile, doing well, tolerating diet
• Contraception plans (must discuss before patient goes home)
• Vaccines – does pt need rubella vaccine prior to discharge?
• Breastfeeding? Problems? Encourage.
• Rhogam, if Rh-negative
• Discharge and follow-up plan
• Patients usually go home if uncomplicated 24-48 hours postpartum
• Follow-up appointment scheduled in 2-6 weeks postpartum
4b.
Sample Postoperative Cesarean Section Orders/Note
Sample C/S Orders
Admit to Recovery Room, then postpartum floor
Diagnosis: Status post (s/p) C/S for failure to progress (FTP)
Condition: Stable
Vitals: Routine, q shift
Allergies: None
Activity: Ambulate with assistance this PM, then up ad lib
Nursing: Strict input and output (I&O), Foley to catheter drainage, call MD for
Temp > 38.4, pulse > 110, BP < 90/60 or > 140/90, encourage breastfeeding,
pad count, dressing checks, and Ted’s leg stockings until ambulating
Diet: Regular as tolerated; some hospitals only allow ice chips or clear liquids
IV: Lactated ringers (LR) or D5LR at 125 cc/hr, with 20 units of Pitocin x 1-2 Liters
Labs: CBC in AM
Medications:
• Morphine sulfate PCA (patient controlled analgesia) per
protocol (1 mg per dose with 10 minute lockout, not to exceed 20 mg/4 hours)
• Percocet 1-2 tabs PO q 4-6 hours prn pain, when tolerating PO well
• Vistaril 25 mg IM or PO q 6 hours prn nausea
• Ibuprofen 800 mg PO q 8 hours prn pain, when tolerating PO well
• Prophylactic antibiotics if indicated
• Thromboprohylaxis for high-risk patients
• Rhogam, if Rh-negative
Sample C/S Note
Date and Time:
Day #1 (Post-op day POD#1)
Subjective: Ask patient about:
• Pain – relieved with medication?
• Nausea/vomiting
• Passing flatus (rare this early post-op)
Objective:
• Vital signs and note tachycardia, elevated or low BP, maximum and current temperature
• Input and output
4
DR.Mojibian
1390
•
Focused physical exam including
o Heart
o Lungs
o Breasts: engorged? Nipples – Is skin intact?
o Incision: Clean and dry, intact?
o Abd: Soft? Location of the uterine fundus – below umbilicus? Firm? Tender?
o Perineum: Assess lochia (blood on pad, how old is pad?)
Visually inspect perineum – Hematoma? Edema? Sutures
intact?
o Extremities: Edema? Cords? Tender?
• Postpartum labs: Hemoglobin or hematocrit
Assessment/Plan: POD#1 status post (S/P) C/S or repeat C/S (indication for the
C/S)
• Afebrile, tolerating pain with medication, oral intake, adequate urine output
(>30cc/hr)
• Routine post-op care
o Discharge Foley
o Discharge PCA or IV pain medications and PO pain Meds when tolerating PO
o Out of bed (OOB)
o Advance diet as tolerated
o Discharge IV when tolerating PO
• Check hematocrit or CBC
5.
Sample Gynecologic History and Physical
Introduction: Name, age, gravidity, parity and presenting problem
HPI:
Past Medical History/Past Surgical
History: Past Gynecologic History:
• Menses – menarche, cycle duration, length, heaviness, intermenstrual
bleeding, dysmenorrhea, and menopause (if relevant).
• Abnormal Pap smears, including time of last Pap
• Sexually transmitted infections
• Sexual history
• Postmenopausal women. Ask about hypoestrogenic symptoms, such as hot flashes or night
sweats, vaginal dryness, and about current and past use of hormone/estrogen replacement
therapy.
• Mammogram
Past OB History: Date of delivery, gestational age, type of delivery, sex, birthweight and any complications
Family
History:
Allergies:
Medication
s: Social
History:
Physical Exam: Complete
Review of
Systems: Plan:
1. Pap smear
2. Endometrial biopsy obtained
3. Medications, etc.
Two Sample Gyn Clinic SOAP Notes
5
DR.Mojibian
1390
S. 22 y/o G2P2 here for annual exam. Regular menses q 28 days with no intermenstrual bleeding. IUD
for contraception since birth of last child 2 years ago. No problems with method. Minimal
dysmenorrhea. Mutually monogamous relationship x 6 years. No hx of abnormal Paps. + BSE, jogs
twice a week, no smoking, no abuse, + seat belts.
O. Breasts: No masses, adenopathy, skin changes
Abd: No masses, soft,
NT Pelvic:
Ext genitalia: Normal
Vagina: pink, moist, well rugated
Cervix: multiparous, no lesions
Bimanual: uterus small, anteverted, NT, no adnexal masses or tenderness
A. Normal exam
P. Pap, RTC 1 year
*
*
*
*
S. 33 y/o G3P1 with LMP 1 week ago here for follow up of chronic left sided pelvic pain. Patient first
seen
6 months ago with complaints of pain x 2 years. She describes pain as dull and aching, intermittent,
with no relationship to eating but increased before and during menses. Pain has gotten worse over the
last 6 months and requires her to miss work 2-3 days per month. No relief with NSAIDs. Patient has
history of
chlamydia 5 years ago for which she was treated. No history of PID. Three partners within the past
year: no condom use No GI symptoms: regular BMs, no constipation, diarrhea, nausea or vomiting.
Past history of ectopic x 2 with removal of part of the left and right tubes. Also had ruptured
appendectomy at age 20. On birth control pills for contraception.
O. Abdomen: 1+ LLQ tenderness, no peritoneal signs
Pelvic: Ext genitalia: Normal
Vagina: no discharge
Cervix: no lesions
Biman: uterus small, retroverted, NT, 3+ left adnexal tenderness, no right adnexal tenderness,
no masses palpated
A. Pelvic pain unresponsive to medical management; rule out endometriosis vs adhesive disease vs
chronic PID vs other
P. Schedule diagnostic laparoscopy
6
DR.Mojibian
6.
1390
Admission Orders
These vary a little from case to case, but the following are fairly general (format is ADC VAN
DISMAL):
Admit:
To the specific service or team
Diagnosis:
List the diagnosis and the names of any associated surgeries or procedures
Condition:
Such as Stable vs Fair vs Guarded
Vitals:
Frequency
Activity:
Ambulation, showering
Nursing:
Foley
catheter
management
parameters Prophylaxis for deep
venous thrombosis
Incentive
spirometry protocols
Call orders
Vital sign parameters for notifying the team
Urine output parameters
Diet:
Oral intake management
IVF:
Rates are typically set at 125 cc per hour
Special:
Drain management
Oxygen management
Meds:
Pain medications
Prophylactic orders, such as for sleep or nausea
The patients' regular medications
Allergies:
Labs:
Typically includes hemoglobin/hematocrit
7
DR.Mojibian
1390
Department of Obstetrics and Gynecology
Massive Transfusion Protocol for Obstetrical
Hemorrhage
I. PRINCIPLE
The Massive Transfusion Protocol (MTP) for Obstetrical Hemorrhage is intended
for antepartum; intrapartum or postpartum patients deemed candidates based on
requirement for massive blood volume replacement. Currently at The University
Hospital, University of Cincinnati, an MTP is in place. This protocol has been
modified to meet the special needs of the obstetrical hemorrhage patient.
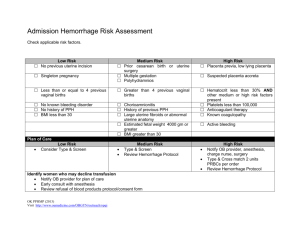
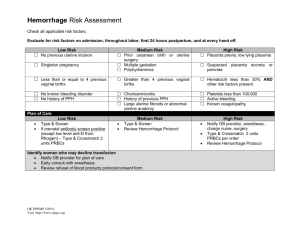
II. CLASSIFICATION OF OBSTETRICAL HEMORRHAGE
A. LOW RISK: Minimal bleeding with reassuring maternal/fetal status.
Vaginal bleeding, which will be expectantly managed.
B. MODERATE RISK: Vaginal bleeding which requires active management.
Transfusion of blood products as well as fetal/maternal intervention may
be necessary.
C. HIGH RISK: Vaginal bleeding which requires active management.
Transfusion of blood products as well as fetal/maternal intervention will be
necessary. A subset of these patients will require the implementation of
the Massive Transfusion Protocol.
1. Antepartum presentation to ER or OB Triage with abruption,
previa or accrete and DIC from any source.
2. Intrapartum hemorrhage immediately following 3rd stage of
labor.
3. Postpartum hemorrhage occurring during recovery period or
on postpartum unit.
III. IMPLEMENTATION OF MTP (HAVE A LOW THRESHOLD FOR INITIATION)
A. Criteria for implementation of MTP (any of below)
1. EBL > 2000 cc with ongoing blood loss of >150 cc/min.
Obstetricians under estimate blood loss. (Refer to Box 1:
Guidelines for Estimation of Blood Loss)
2. Hypotension decrease of BP by 20% in the setting of acute
hemorrhage
3. Tachycardia HR >110 in the setting of acute hemorrhage
4. Mental status changes in the setting of acute hemorrhage
5. Chest pain/EKG changes in the setting of acute hemorrhage
8
6. O2 Saturation <95% with O2 treatment in the setting of acute
hemorrhage or significant change in O2 saturation
7. Prior to the onset of hemorrhage in special cases. (See
Section IV: INITIATION OF THE MTP PRIOR TO THE
ONSET OF VISIBLE HEMORRHAGE)
8. Absence/Decrease in urine output.
9. INR > 1.5
10. Temp < 96.5
11. Base Deficit > -6.0
B. Acceptable scenarios
1. Known accreta
2. Known previa with greater than 2 prior cesarean sections.
3. Strong suspicion of large concealed abruption.
4. Active bleeding disorder at the time of operative delivery
5. In other cases, where there is a very high likelihood for
massive obstetrical hemorrhage, the MTP can be
implemented prior to a scheduled delivery.
Box 1.
Guidelines for Estimation of Blood Loss
Suction Canister
As measured
(Side pockets must be suctioned during
C/S for accurate measurement)
Saturated Laparotomy Pad
# Laps x 100 cc
Unsaturated Laparotomy Pad
# Laps x 50 cc
Estimation of Amniotic Fluid
Amount
subtracted as
estimated by
surgeon
C. Notification of personnel – OB charge nurse (513-584-5422)
1. The following personnel will be notified at the time criteria has
been met for implementation of the MTP
a. OB Attending (513-325-0304)
b. Anesthesia Attending and Team (513-314-2969)
c. Notify blood bank of situation (513-584-7888)
d. OB Tech (to set up OB OR if necessary)(513-5845740/5741)
2. Call for backup (OB Attending, Gyn Onc [Dr. Richards 513432-2614 Cell], MFM [513-504-6099], Trauma Surgeons [513994-0911])
D. Decision for implementation
1. The decision to implement the MTP must be a joint decision
by the OB and Anesthesia attending
E. Laboratory Evaluation: (Label, “OR-Obstetrical Crash”)
1. At the implementation of the MTP the following laboratory
studies will be sent.
a. Type and Match (if not current) (pink tube)
b. CBC with Platelets (purple tube)
c. PT/PTT/INR (blue tube)
d. Fibrinogen (blue tube)
e. D-Dimer (blue tube)
e. Renal panel, Ionized Calcium, Magnesium (serum
separator or red top), Phosphorous
f. ABG (blood gas kit)
g. VBG (blood gas kit)
h. Lactic Acid (grey on ice)
2. This series of laboratory studies will be known as the MTP
Lab Panel.
3. The MTP Lab Panel will be sent at the initiation of the MTP
and Q 2 hours thereafter until the MTP has been terminated.
F. Patient Preparation
1. The patient must have at least 2 - 18G peripheral IV’s
2. The patient must be relocated to a room with capability for
central monitoring. Preferably, if the patient is in a labor
room she should be transferred to Obstetrical OR Room 4, if
possible.
3. Foley catheter will be placed.
4. Continuous pulse oximetry and EKG monitoring.
5. Bair Hugger to maintain Normothermia
6. All intravenous fluids must be warmed through fluid
warmers.
7. OR suite must be warmed to 27° Celsius (-80˚F).
IV. PROCEDURE FOR IMPLEMENTATION OF MASSIVE TRANSFUSION
PROTOCOL
A. Notification of Transfusion Services (Obstetrical MTP)
1. The Blood Bank/Crossmatch Lab will be notified of the
implementation of the MTP by ONLY one of the following
responsible individuals: Attending OB, Attending
Anesthesiologist, Chief Resident/Charge-circulating nurse
2. The Blood Bank/Crossmatch Lab will be notified via
telephone at 513-584-7888.
3. The patient name, MR #, and patient location must be
communicated to the Compatibility/Crossmatch Lab.
4. It is best to say, “I would like to implement the Massive
Transfusion Protocol for Obstetrical Hemorrhage…”
5. It is the responsibility of the contacting individual to
ensure the appropriate blood specimen has been
collected and sent to the Transfusion Service. Of note,
most obstetrical
patients will have a current Type and Screen and
this specimen may not be necessary. Verify that
type and screen is current.
6. In acute situations where there is no current Type and
Screen, uncross matched O negative trauma blood
may be used (location: blood bank 6th floor or main OR
refrigerator). This must be authorized by signature on
arrival of the blood products by the OB or Anesthesia
Attending.
B. Acceptable Blood Specimen (Label “OR-Obstetrical Crash”)
1. EDTA (purple or pink) tube appropriately labeled
and initialed for blood bank. (PREFERRED)
2. SST, plain red top tube. (ACCEPTABLE)
3. CORVAC tube, hemolyzed specimen, improperly
labeled specimen is UNACCEPTIBLE.
4. Specimen may have been stored at 2-8° C for </= 3
days.
5. Nurse needs to label with patient sticker and dated,
timed and signed with initials
C. Transfusion Service Personnel Duties
1. Perform ABO, Rh typing on specimen.
2. Prepare products per protocol. See Box 2.
3. Continue preparation of new products until
protocol is terminated (See Section V. H. :
Termination of MTP)
4. Notify the charge nurse area that the products are
available.
5. Products will be stored in a cooler for storage and
transport.
Box 2.
Cycle #
RBC
Plasma
Platelets
Cryo
Massive Transfusion Protocol for Obstetrical Hemorrhage
Cooler #1 contents
Cooler #2 contents
Cooler #3 Contents
6 units P RBC
6 units P RBC
6 units P RBC
4 units FFP
4 units FFP
4 units FFP
5 units pooled or 1
5 units pooled or 1
5 units pooled or 1
aphaeresis product
aphaeresis product
aphaeresis product
10 Pooled Cryo
10 Pooled Cryo
Consider recombinant fader VIIa from
Pharmacy IV
room 513-584-8847
D. Obtaining Products
1. The OB tech or charge nurse designee will act as the
runner to collect the products from the Blood Bank:
Room 6158 of University Hospital, 6th floor.
E. Transfusion of Products
1. All transfusion, except platelets, will be performed
using the Level 1 Fluid Warmer/Infuser
2. Transfusion will proceed in the following order:
a. 2 units
PRBC
b. 2 units
FFP
c. 5 units pooled
platelets d. 2-3 units
PRBC
e. 2 units
FFP
f. Repeat Steps a
-e
g. 10 pooled cryoprecipitate
h. Repeat steps f & g as frequently as deemed
necessary based on patient’s status and active
blood loss
F. Documentation
1. Documentation will be maintained during the
execution of the MTP including the following items:
a. Vital signs Q 10 minutes (Temp, BP, HR, RR)
b. Pulse
oximetry c.
Urine output
d. Products
administered
e. Time of administration of
products f.
Medications
administered
g. Time of medication
administration h. Laboratory
studies
2. It will be the responsibility of the anesthesia team to
maintain the above documentation in the OR (as noted in
the anesthesia record) and by the charge nurse if patient
is in
the PACU or preoperative
area.
3. All blood transfusion slips will be filed and attached to
the chart of the patient by the RN/Anesthesia.
G. Termination of MTP
1. The termination of the MTP will only be determined by
one of the following responsible individuals: Attending Ob
or Attending Anesthesiologist.
2. The Blood Bank/Crossmatch Lab will be notified of
the termination of the MTP by ONLY one of the
following responsible individuals: Attending OB,
and Attending Anesthesiologist, charge nurse.
3. All patients who go to the SICU will be transferred to the
Trauma Service. Once on that service, they determine
when to stop the protocol.
4. The Blood Bank/Crossmatch Lab will be notified
via telephone at 513-584-7888.
5. Call for SICU bed 584-4433.
perinatology.com
OBPharmacopoeia
Home > Reference > OBPharmacopoeia TOC-Public> Pospartum hemorrhage algorithm
Postpartum Hemorrhage Algorithm
The following algorithm is based the California Maternal Quality Care Collaborative OB Hemorrhage Protocol.
Stage 0
Blood Loss less than 500ml with Vaginal delivery; less than 1000 ml with cesarean section. Stable vital signs
All women receive active management of 3rd stage Oxytocin IV infusion or 10 Units IM
Vigorous fundal massage for 15 seconds minimum
Stage 1
Blood Loss > 500ml Vaginal delivery; > 1000 ml cesarean section
15% Vital Sign change -or-HR equal to or greater than 110, BP equal to or less than 85/45 O2 Sat less than 95%,
pallor, delayed capillary refill, or decreased urine output. can indicate
Decreased urine output, decreased BP and tachycardia may be late signs of compromise
Call for help.
Provide adequate ventilation
Assist airway protection
Establish large-bore intravenous access
Supplemental O2 5-7 L/min by tight face mask
Prepare 2 units of packed red cells.
Evaluate for atony, lacerations, hematoma, inverted uterus , retained tissue, accreta, coagulopathy.
o
Medication for uterine atony
Oxytocin
10-40 units in 1 liter NS or LR IV rapid infusion
o
Methylergonovine (Methergine)
0.2 milligrams intramuscular q 2-4 hrs up to 5 doses
Stage 2
1000-1500 ml estimated blood loss with continued bleeding.
o
Move to operating room
Transfuse 2 Units PRBCs per clinical signs
Consider thawing 2 Units FFP
Order CBC, PT/INR/PTT, Fibrinogen
Warm blood products and infusions to prevent hypothermia, coagulopathy and arrhythmias
Medication for uterine atony
o
Prostaglandin F2 Alpha (Hemabate)
250 micrograms intramuscular, intramyometrial, repeat q 15-90 minutes,
maximum 8 doses
o
Prostaglandin E2 suppositories (Dinoprostone, Prostin E2)
20 milligrams per rectum q 2 hrs
o
Misoprostol (Cytotec)
1000 micrograms per rectum
Surgical intervention
Vaginal Birth:
Atony Bimanual Fundal Massage
Retained POC: Dilation and Curettage
Lower segment/Implantation site/Atony: Intrauterine Balloon
Laceration/Hematoma: Packing, Repair as Required
Cesarean Birth:
Continued Atony: B-Lynch Suture/Intrauterine Balloon
Continued Hemorrhage: Uterine Artery Ligation
Hypogastric Ligation (experienced surgeon only)
o
o
o
o
o
o
o
o
Stage 3
Estimated blood loss gretaer than 1500 ml with continued blood loss.
o
Activate massive transfusion protocol (MTP),
MTP "Pack", to be sent from the Blood Bank is:
4 units PRBC
2 OR 4 units FFP
1 apheresis pack of platelets
o
Obtain CBC , PT/INR/PTT, and fibrinogen every 4 hours after the standard MTP "Pack" is given. Laboratory studies
should be monitored for at least 24 hours after discontinuing the protocol.
Note: 10 units cryoprecipitate should be given for fibrinogen <100mg/dl
o
If bleeding continues after 2 MTP packs have been administered, or women is refusing transfusions (e.g. Jehovah
Witnesses) , consider recombinant activated factor VII (rFVIIa, NovoSeven®) 60 mcg/kg. May repeat in 30 minutes
Surgical intervention
o B-Lynch Suture/Intrauterine Balloon
o
o
o
Uterine Artery Ligation
Hypogastric Ligation (experienced surgeon only)
Hysterectomy
REFERENCES:
1.http://www.cmqcc.org/resources/ob_hemorrhage/ob_hemorrhage_protocol_tools_release_1_2
2. Burtelow M, Riley E, Druzin M, Fontaine M, Viele M, Goodnough LT. How we treat: management of life-threatening primary
postpartum hemorrhage with a standardized massive transfusion protocol. Transfusion. 2007 Sep;47(9):1564-72. PMID: 17725718
3. Malone , MD , LTC USAF, SGRS, D.L., Hess , MD , MPH, J. R., & Fingerhut, MD, A., (2006). Massive Transfusion Practices
Around the Globe and a Suggestion for Common Massive Transfusion Protocol. J Trauma , 60, S91-S96.
4. Sobieszczyk S, Breborowicz GH. The use of recombinant factor VIIa in A Textbook of PostPartum Hemorrhage (ed C. B-Lynch et
al.). Sapiens Publishing 2006; p 250
5. Franchini M, Franchi M, Bergamini V, Salvagno GL, Montagnana M, Lippi G. A Critical Review on the Use of Recombinant
Factor VIIa in Life-Threatening Obstetric Postpartum Hemorrhage. Semin Thromb Hemost 2008; 34: 104-12.
6. O'Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human
coagulation factor VIIa. JAMA 2006; 295: 293-8.
7. Hemabate (carboprost tromethamine) package insert. Pharmacia & Upjohn Co., Division of Pfizer Inc, NY, NY 10017. FDA rev
date: march 2006
8. . O'Brien P, et al. Rectally administered misoprostol for the treatment of postpartum hemorrhage unresponsive to oxytocin and
ergometrine: a descriptive study.Obstet Gynecol. 1998 Aug;92(2):212-4. PMID: 9699753
9. Methergine package insert. Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936. FDA Rev date: 4/26/2007
THE INFORMATION IN THE OBPHARMACOPOEIATM IS INTENDED SOLELY FOR USE BY THE MEDICAL PROFESSION.
IT IS NOT INTENDED FOR LAY PERSONS.
FOCUS INFORMATION TECHNOLOGY, INC. DOES NOT ASSUME ANY RESPONSIBILITY FOR ANY ASPECT OF
HEALTHCARE ADMINISTERED WITH THE AID OF THIS CONTENT. THE PRESCRIBING PHYSICIAN MUST BE
FAMILIAR WITH THE FULL PRODUCT LABELING AS PROVIDED BY THE MANUFACTURER AND RELEVANT
MEDICAL LITERATURE PRIOR TO USING THE OBPHARMACOPOEIATM .
Home | About | Disclaimer | Privacy | Contact
Copyright © 2009 by Focus Information Technology.
All rights reserved
perinatology.com
OBPharmacopoeia
Home > Reference > OBPharmacopoeia TOC-Public> Pospartum hemorrhage algorithm
Postpartum Hemorrhage Algorithm
The following algorithm is based the California Maternal Quality Care Collaborative OB Hemorrhage Protocol.
Stage 0
Blood Loss less than 500ml with Vaginal delivery; less than 1000 ml with cesarean section. Stable vital signs
All women receive active management of 3rd stage Oxytocin IV infusion or 10 Units IM
Vigorous fundal massage for 15 seconds minimum
Stage 1
Blood Loss > 500ml Vaginal delivery; > 1000 ml cesarean section
15% Vital Sign change -or-HR equal to or greater than 110, BP equal to or less than 85/45 O2 Sat less than 95%,
pallor, delayed capillary refill, or decreased urine output. can indicate
Decreased urine output, decreased BP and tachycardia may be late signs of compromise
Call for help.
Provide adequate ventilation
Assist airway protection
Establish large-bore intravenous access
Supplemental O2 5-7 L/min by tight face mask
Prepare 2 units of packed red cells.
Evaluate for atony, lacerations, hematoma, inverted uterus , retained tissue, accreta, coagulopathy.
o
Medication for uterine atony
Oxytocin
10-40 units in 1 liter NS or LR IV rapid infusion
o
Methylergonovine (Methergine)
0.2 milligrams intramuscular q 2-4 hrs up to 5 doses
Stage 2
1000-1500 ml estimated blood loss with continued bleeding.
o
Move to operating room
Transfuse 2 Units PRBCs per clinical signs
Consider thawing 2 Units FFP
Order CBC, PT/INR/PTT, Fibrinogen
Warm blood products and infusions to prevent hypothermia, coagulopathy and arrhythmias
Medication for uterine atony
o
Prostaglandin F2 Alpha (Hemabate)
250 micrograms intramuscular, intramyometrial, repeat q 15-90 minutes,
maximum 8 doses
o
Prostaglandin E2 suppositories (Dinoprostone, Prostin E2)
20 milligrams per rectum q 2 hrs
o
o
o
o
o
o
o
o
o
Misoprostol (Cytotec)
1000 micrograms per rectum
Surgical intervention
Vaginal Birth:
Atony Bimanual Fundal Massage
Retained POC: Dilation and Curettage
Lower segment/Implantation site/Atony: Intrauterine Balloon
Laceration/Hematoma: Packing, Repair as Required
Cesarean Birth:
Continued Atony: B-Lynch Suture/Intrauterine Balloon
Continued Hemorrhage: Uterine Artery Ligation
Hypogastric Ligation (experienced surgeon only)
Stage 3
Estimated blood loss gretaer than 1500 ml with continued blood loss.
o
Activate massive transfusion protocol (MTP),
MTP "Pack", to be sent from the Blood Bank is:
4 units PRBC
2 OR 4 units FFP
1 apheresis pack of platelets
o
Obtain CBC , PT/INR/PTT, and fibrinogen every 4 hours after the standard MTP "Pack" is given. Laboratory studies
should be monitored for at least 24 hours after discontinuing the protocol.
Note: 10 units cryoprecipitate should be given for fibrinogen <100mg/dl
o
If bleeding continues after 2 MTP packs have been administered, or women is refusing transfusions (e.g. Jehovah
Witnesses) , consider recombinant activated factor VII (rFVIIa, NovoSeven®) 60 mcg/kg. May repeat in 30 minutes
Surgical intervention
o B-Lynch Suture/Intrauterine Balloon
o Uterine Artery Ligation
o Hypogastric Ligation (experienced surgeon only)
o Hysterectomy
REFERENCES:
1.http://www.cmqcc.org/resources/ob_hemorrhage/ob_hemorrhage_protocol_tools_release_1_2
2. Burtelow M, Riley E, Druzin M, Fontaine M, Viele M, Goodnough LT. How we treat: management of life-threatening primary
postpartum hemorrhage with a standardized massive transfusion protocol. Transfusion. 2007 Sep;47(9):1564-72. PMID: 17725718
3. Malone , MD , LTC USAF, SGRS, D.L., Hess , MD , MPH, J. R., & Fingerhut, MD, A., (2006). Massive Transfusion Practices
Around the Globe and a Suggestion for Common Massive Transfusion Protocol. J Trauma , 60, S91-S96.
4. Sobieszczyk S, Breborowicz GH. The use of recombinant factor VIIa in A Textbook of PostPartum Hemorrhage (ed C. B-Lynch et
al.). Sapiens Publishing 2006; p 250
5. Franchini M, Franchi M, Bergamini V, Salvagno GL, Montagnana M, Lippi G. A Critical Review on the Use of Recombinant
Factor VIIa in Life-Threatening Obstetric Postpartum Hemorrhage. Semin Thromb Hemost 2008; 34: 104-12.
6. O'Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human
coagulation factor VIIa. JAMA 2006; 295: 293-8.
7. Hemabate (carboprost tromethamine) package insert. Pharmacia & Upjohn Co., Division of Pfizer Inc, NY, NY 10017. FDA rev
date: march 2006
8. . O'Brien P, et al. Rectally administered misoprostol for the treatment of postpartum hemorrhage unresponsive to oxytocin and
ergometrine: a descriptive study.Obstet Gynecol. 1998 Aug;92(2):212-4. PMID: 9699753
9. Methergine package insert. Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936. FDA Rev date: 4/26/2007
THE INFORMATION IN THE OBPHARMACOPOEIATM IS INTENDED SOLELY FOR USE BY THE MEDICAL PROFESSION.
IT IS NOT INTENDED FOR LAY PERSONS.
FOCUS INFORMATION TECHNOLOGY, INC. DOES NOT ASSUME ANY RESPONSIBILITY FOR ANY ASPECT OF
HEALTHCARE ADMINISTERED WITH THE AID OF THIS CONTENT. THE PRESCRIBING PHYSICIAN MUST BE
FAMILIAR WITH THE FULL PRODUCT LABELING AS PROVIDED BY THE MANUFACTURER AND RELEVANT
MEDICAL LITERATURE PRIOR TO USING THE OBPHARMACOPOEIATM .
Home | About | Disclaimer | Privacy | Contact
Copyright © 2009 by Focus Information Technology.
All rights reserved
WHO guidelines
for the management of
postpartum haemorrhage
and retained placenta
WHO guidelines
for the management of
postpartum haemorrhage and
retained placenta
summarized in the GRADE tables. However, they were mentioned in the evidence
summary and taken into account in the recommendation. GRADE tables were not
prepared for case series or reports.
The draft GRADE tables were reviewed by the WHO Secretariat together with CREP
staff. Recommendations relating to the questions and outcomes proposed were then
drafted ahead of the Technical Consultation. A draft of the methodology, results, and
recommendations was sent for review to a subgroup of the experts participating in
the Technical Consultation before the meeting.
Decision-making during the technical consultation
For each question, the participants in the Technical Consultation discussed the draft
text prepared by the Secretariat, with the aim of reaching a consensus. Consensus
was defined as agreement by the majority of participants, provided that those who
disagreed did not feel strongly about their position. Any strong disagreements were
recorded as such.
During the meeting, in addition to the documentation prepared by the Secretariat,
preliminary results from four unpublished trials were made available. While the
presentation of the most recent data from large trials was welcomed and used to
inform the recommendations, some participants expressed a need for more time
to review these results before making recommendations. The GRADE tables in this
document include evidence from the search as well as the data presented and
discussed during the Technical Consultation.
The system used to establish the strength and ranking of the recommendations
involved assessing each intervention on the basis of: (i) desirable and undesirable
effects; (ii) quality of available evidence; (iii) values and preferences related to
interventions in different settings; and (iv) cost of options available to health care
workers in different settings.
Scope of the guidelines
The draft questions and list of outcomes related to the treatment of PPH and
management of retained placenta were sent to 144 experts from all parts of the
world. Responses were received from 60 of these experts: 46 physicians,
7 midwives, and 7 non-clinicians (policy-makers, researchers and consumers),
representing all 6 WHO regions.
There were 39 questions in 6 domains:
▪
▪
▪
assessment of blood loss (1 question);
drugs for atonic PPH (13 questions);
non-drug interventions for atonic PPH:
–
–
–
mechanical (6 questions);
radiological (1 question);
surgical (8 questions);
▪
▪
▪
retained placenta (4 questions);
organizational and educational interventions (5 questions);
crystalloid versus colloid fluids for resuscitation (1 question). This question was
included following a suggestion from the respondents during the survey.
The average scores for questions and outcomes are shown in Annex 1.
It should be noted that not all outcomes are applicable to all questions. As mentioned
above, questions that scored less than 7 are also included in the guidelines.
Evidence and recommendations
A. Diagnosis of PPH
1. Should blood loss be routinely quantified during management of
the third stage of labour for the purpose of diagnosing PPH?
Several related studies that looked at measurement of blood loss following childbirth,
with the objective of ensuring timely diagnosis of PPH and improving health
outcomes, were assessed. No study was found that directly addressed the question.
Summary of evidence
Visual versus quantitative methods for estimating blood loss after vaginal delivery
One RCT (3) compared visual estimation of blood loss with measurement of
blood collected in a plastic drape. Six observational studies (4–9), with a total of
594 participants, compared visual estimation with known values in the delivery
room or in simulated scenarios. Three studies (10–12) compared visual or quantified
estimations with laboratory measurement in 331 vaginal deliveries.
In the RCT (3), visual estimation underestimated blood loss when compared with
drape measurement (mean difference 99.71 ml) (page 27, GRADE Table A1). Visual
methods underestimated blood loss when compared with known simulated volumes.
Training courses on estimating blood loss after vaginal delivery
One RCT (13) compared the accuracy of estimation of blood loss by 45 nurses
attending a course on blood loss estimation and 45 nurses not attending the course.
In this small RCT (13), with seven simulated scenarios, blood loss was accurately
estimated by 75.55% of the nurses attending a training course compared with 24.44%
without training (relative risk (RR) 3.09; 95% confidence interval (CI) 1.80–5.30)
(page 27, GRADE Table A2).
In three studies (14–16), a total of 486 staff members of maternity services visually
estimated blood loss in simulated scenarios before and after training courses. The
three uncontrolled studies (14–16) show results in the same direction as the RCT.
Recommendation
After childbirth, blood loss and other clinical parameters should be closely monitored.
At present, there is insufficient evidence to recommend quantification of blood loss
over clinical estimation. (Quality of evidence: low. Strength of recommendation:
strong)
Remarks
The participants identified several priority research topics related to the definition
and diagnosis of PPH.
▪
What quantity of blood loss should be the marker for diagnosis of PPH?
▪
Does the act of quantifying blood loss alter (or lead to improved) clinical outcomes
for the mother and her baby?
▪
Which clinical consequences of blood loss are of greatest value for the diagnosis
and treatment of PPH?
B. Management of atonic PPH
As a general preventive approach, the use of oxytocin for active management of the
third stage of labour is strongly recommended, because it reduces PPH by more than
60% (17).
1. Medical interventions for management of PPH
The Consultation was asked to assess the value of injectable uterotonics (oxytocin,
ergometrine, fixed dose combination of oxytocin and ergometrine, carbetocin and
injectable prostaglandin), misoprostol (tablet form used via oral, sublingual and
rectal routes), injectable tranexamic acid and injectable recombinant factor VIIa in
the management of PPH thought to be due to uterine atony.
For oxytocin, ergometrine and prostaglandin F2α, the Consultation agreed with the
doses recommended in the WHO guide, Managing complications in pregnancy and
childbirth (18), as given in Table 1 (overleaf).
The recommendations below may also be used in cases of PPH due to uterine
atony following caesarean section. The Consultation acknowledged that these
recommendations were based primarily on data following vaginal birth, and that
specific data on PPH due to uterine atony following caesarean section were scarce
and not evaluated separately from data on vaginal births.
(a) Which uterotonics should be offered in the management of PPH
due to uterine atony?
Summary of evidence
Except for the specific misoprostol trials evaluated in section (b), the evidence has
been extrapolated from research on prevention of PPH. Systematic reviews
comparing the effects of oxytocin with ergometrine (19), a fixed-dose combination
of oxytocin and ergometrine (20), carbetocin (21) and prostaglandins (22) in the
prevention of PPH were reviewed. The guidelines on prevention of postpartum
haemorrhage published by WHO synthesized and graded the evidence and made
recommendations (1). That publication includes the relevant GRADE tables.
Separate GRADE tables were not prepared for this question and the evidence is
summarized below narratively. One RCT comparing oxytocin to ergometrine in
600 women (23) was published subsequent to the systematic review and publication
of the WHO guidelines.
Table 1. Drug doses for management of PPH
Oxytocin
Ergometrine/
Methyl-ergometrine
15-Methyl
prostaglandin F2a
Dose and route
IV: Infuse 20 units in 1 l IM or IV (slowly): 0.2 mg
IV fluids at 60 drops per
minute
IM: 0.25 mg
Continuing dose
IV: Infuse 20 units in 1 l Repeat 0.2 mg IM after
IV fluids at 40 drops per 15 minutes
minute
If required, give 0.2 mg
IM or IV (slowly every
4 hours
0.25 mg every
15 minutes
Maximum dose
Not more than 3 l of
IV fluids containing
oxytocin
5 doses (Total 1.0 mg)
8 doses (Total 2 mg)
Precautions/
contraindications
Do not give as an IV
bolus
Pre-eclampsia,
hypertension, heart
disease
Asthma
Prostaglandin F2a should not be given intravenously. It may be fatal. Managing complications in
pregnancy and childbirth. Geneva, World Health Organization, 2000, page S-28, table S–8.
IV intravenous
IM intramuscular
Oxytocin vs ergometrine
One trial (24) included in the systematic review reported on the critical outcomes
of blood loss of >1000 ml and need for blood transfusion. There was no difference
in incidence of blood loss >1000 ml (RR 1.09, 95%CI 0.45–2.66). Blood transfusion was
given to 2 of 78 women receiving oxytocin compared with 1 of 146 women receiving
ergometrine (RR 3.74, 95%CI 0.34–40.64). No significant difference was observed in
the use of additional uterotonics in two trials in the systematic review (24, 25): in
35 of 557 women given oxytocin and 46 of 651 women given ergometrine (RR 1.02,
95% CI 0.67–1.55).
In the later Nigerian trial (23), the use of additional uterotonics was reported in
18 of 297 patients receiving oxytocin in the third stage of labour compared with
30 of 303 receiving ergometrine (RR 0.61, 95%CI 0.35–1.07). The incidence of adverse
side-effects was significantly lower in women receiving oxytocin than in those given
ergometrine; for vomiting, the RR was 0.09 and the 95% CI 0.05–0.16); for elevated
blood pressure, RR was 0.01 and 95% CI 0.00–0.15).
Oxytocin-ergometrine fixed dose combination vs oxytocin
With regard to blood loss >1000 ml, decreased blood loss was observed in the
group given the fixed-dose combination of oxytocin (5 IU) and ergometrine (0.5 mg)
although the difference was not statistically significant (Peto odds ratio (OR) 0.78,
95%CI 0.58–1.03). In four studies that reported on the use of blood transfusion, there
was no significant difference and wide confidence interval compatible with either
direction of effect (Peto OR 1.37, 95%CI 0.89–2.10). Three studies reported a slight,
but statistically significant, lower use of additional uterotonics in the group receiving
fixed dose oxytocin-ergometrine combination (RR 0.83, 95%CI 0.72–0.96). Four studies
reported on the incidence of side-effects, notably a higher incidence of elevated
diastolic blood pressure in the group given the oxytocin-ergometrine fixed dose
combination (RR 2.40, 95%CI 1.58–3.64).
Oxytocin-ergometrine fixed dose combination vs ergometrine
None of the critical outcomes was addressed in the studies.
Carbetocin vs oxytocin
No data on blood loss ≥1000 ml, blood transfusion or surgical treatments were
available. For the other priority outcomes, the use of additional uterotonics was
similar in the two groups (RR 0.93, 95%CI 0.44–1.94), but there was less use of uterine
massage in the carbetocin group (RR 0.70, 95% CI 0.51–0.94). Data on side-effects
were too limited to allow any judgements to be made (nausea: RR 0.66, 95%CI
0.22–2.00; vomiting: RR 0.07, 95%CI 0.00–1.25; headache: RR 0.51, 95%CI 0.20–1.30).
Carbetocin vs Oxytocin-ergometrine fixed dose combination
Of 150 women given carbetocin and 150 given oxytocin-ergometrine fixed dose
combination, only one woman given the combination experienced blood loss ≥1000 ml
(26). Use of additional uterotonics was similar, with wide confidence intervals (RR 1.3,
95%CI 0.56–3.13), but the occurrence of side-effects was lower in the carbetocin
group (nausea: RR 0.18, 95%CI 0.04–0.78; hypertension up to 60 minutes postpartum:
RR 0.11, 95%CI 0.03–0.47). In a smaller observational study (27), fewer women in the
carbetocin group had a blood loss of >1000 ml (1 of 55 given carbetocin and 9 of 62
given the combination (RR 0.12, 95%CI 0.15–0.94)).
Intramuscular prostaglandins vs injectable uterotonics)
No difference was observed in the risk of blood transfusion between these two
treatments (RR 1.05, 95%CI 0.39–2.86). Use of additional uterotonics was not
significantly different between the prostaglandin group (4 of 106) and the injectable
uterotonic group (2 of 116) (RR 2.05, 95%CI 0.39–10.92). Vomiting was observed in
15 of 103 patients receiving prostaglandin and 1 of 107 patients receiving injectable
uterotonics (RR 10.74, 95%CI 2.06–56.02).
Sulprostone vs injectable uterotonics
Two RCTs conducted in the Netherlands (28, 29) reported on estimated blood loss
of ≥1000 ml. There was a nonsignificant reduction in the risk of severe PPH in both
low-risk (28) and high-risk women (29) (RR 0.41, 95%CI 0.14–1.20) in women receiving
sulprostone. The Van Selm study (29) was terminated early because of concerns
regarding myocardial infarctions in women treated with sulprostone and mifepristone.
Carboprost vs misoprostol
No evidence was found relating to the priority outcomes regarding blood loss. Of
60 patients in the carboprost group, none received a blood transfusion compared
with 1 of 60 in the misoprostol group (RR 0.33, 95%CI 0.01–8.02) (30). No patients in
the carboprost group reported shivering, compared with 5 in the misoprostol group
(RR 0.09, 95%CI 0.01–1.61).
Misoprostol vs injectable uterotonics
When compared with injectable uterotonics there was an increase in the risk of
blood loss of ≥1000 ml in women receiving oral misoprostol (400–800 μg) (RR 1.32,
95%CI 1.16–1.51), but no statistically significant difference in the incidence of severe
morbidity, including maternal death (RR 1.00, 95%CI 0.14–7.10). These trials did not
report the outcome of invasive or surgical treatments.
Recommendations
▪
For management of PPH, oxytocin should be preferred over ergometrine
alone, a fixed-dose combination of ergometrine and oxytocin, carbetocin,
and prostaglandins. (Quality of evidence: very low to low. Strength of
recommendation: strong.)
▪
If oxytocin is not available, or if the bleeding does not respond to oxytocin,
ergometrine or oxytocin-ergometrine fixed-dose combination should be offered
as second-line treatment. (Quality of evidence: very low to low. Strength of
recommendation: strong.)
▪
If the above second-line treatments are not available, or if the bleeding does
not respond to the second-line treatment, a prostaglandin should be offered as
the third line of treatment. (Quality of evidence: very low to low. Strength of
recommendation: strong.)
Remarks
▪
The above recommendations are based largely on data from prevention trials or
case series. However, data from treatment RCTs were available for misoprostol
versus oxytocin.
▪
The pharmacokinetics, bioavailability and mode of action of oxytocin and
ergometrine and the uterotonic effects of misoprostol in other obstetric and
gynaecological uses were considered by the participants in the Consultation in
making the recommendations.
▪
Misoprostol may be considered as a third line of treatment for the management of
PPH, because of its ease of administration and low cost compared with injectable
prostaglandins (see also section (b)).
▪
The Consultation noted that the cost of carbetocin was high compared with the
other options. Moreover, it found no evidence that carbetocin has a significant
advantage over oxytocin.
(b) Should misoprostol be offered in the management of PPH due to
uterine atony?
The Consultation made recommendations relating to two separate scenarios: women
who received prophylactic oxytocin during the third stage of labour and those who
did not.
(i) Should misoprostol be offered for the management of PPH in women who
have received prophylactic oxytocin during the third stage of labour?
Summary of evidence
Four trials assessed the use of misoprostol as an adjunct following active
management of the third stage of labour with oxytocin (31–34). The three published
trials (31–33) were relatively small, with a total of 465 women participating. The
unpublished WHO-Gynuity trial (34) included 1400 women in Argentina, Egypt, South
Africa, Thailand and Viet Nam. In three trials (31, 33, 34), 600 µg of misoprostol
was administered orally or sublingually, while in the fourth trial (32) 1000 µg was
administered orally, sublingually or rectally. The results of the WHO-Gynuity trial (34)
were presented to the Consultation and are included in the GRADE table (page 28,
GRADE Table B1).
Taken altogether, when misoprostol as an adjunct was compared with placebo in
women receiving other standard treatments, there were no statistical differences
in the critical outcomes of additional blood loss ≥500 ml (RR 0.83, 95%CI 0.64–1.07),
additional blood loss ≥1000 ml (RR 0.76, 95%CI 0.43–1.34) and blood transfusion
(RR 0.96, 95%CI 0.77–1.19). Similarly, in the large WHO-Gynuity trial (34), the critical
outcomes of additional blood loss ≥500 ml (RR 1.01, 95%CI 0.78–1.30), additional blood
loss ≥1000 ml (RR 0.76, 95%CI 0.43–1.34) and blood transfusion (RR 0.89, 95%CI 0.69–
1.13) were not clinically or statistically significantly different in the two groups.
Recommendation
There is no added benefit of offering misoprostol as adjunct treatment for PPH in
women who have received oxytocin during the third stage of labour. Where oxytocin
is available, and is used in the management of the third stage of labour, oxytocin
alone should be used in preference to adjunct misoprostol for the management
of PPH. (Quality of evidence: moderate to high. Strength of recommendation:
strong.)
Remark
The recommendation is based mainly on data from one large unpublished randomized
controlled trial (34).
(ii) Should misoprostol be offered as a treatment for PPH in women who did not
receive prophylactic oxytocin during the third stage of labour?
Summary of evidence
The evidence relating to this question came from one large RCT conducted in
Ecuador, Egypt and Viet Nam (35), which compared 800 µg of misoprostol given
sublingually with 40 IU of oxytocin given intravenously. Unpublished trial results
were presented to the Consultation (page 29, GRADE Table B2). Women who received
misoprostol had a significantly increased risk of additional blood loss ≥500 ml
(RR 2.66, 95%CI 1.62–4.38) and of needing additional therapeutic uterotonics (RR 1.79,
95%CI 1.19–2.69). There were few cases of additional blood loss ≥1000 ml (5 of 488 in
the group given misoprostol and 3 of 489 given oxytocin). There was an increased risk
of blood transfusion in the misoprostol group, of borderline statistical significance
(RR 1.54, 95%CI 0.97–2.44).
Regarding side-effects, 66 of 488 women receiving misoprostol had a body
temperature above 40 °C, compared with none of 490 given oxytocin. Most of the
cases of high temperature occurred in Ecuador, where 36% of the women given
misoprostol had a temperature above 40 °C. There were no cases in Egypt. Seven of
the women with high temperature had delirium.1
Recommendation
In women who have not received oxytocin as a prophylactic during the third stage
of labour, oxytocin alone should be offered as the drug of choice for the treatment
of PPH. (Quality of evidence: moderate to high. Strength of recommendation:
strong.)
Remarks
▪
Evidence of the superiority of oxytocin over misoprostol for the treatment of PPH
came from one large trial, which showed oxytocin to have higher effectiveness
and fewer side-effects.
▪
The Consultation recognized that oxytocin may not be available in all settings.
It encouraged health care decision-makers in these settings to strive to make
oxytocin and other injectable uterotonics available. However, because the use of
a uterotonic is essential for the treatment of PPH due to atony, it considered that
misoprostol may be used until oxytocin can be made available.
▪
The Consultation noted that the doses of misoprostol used in the trials on
prevention of PPH ranged from 200 µg to 800 µg, administered orally, sublingually
or rectally. In the PPH treatment trials, doses from 600 µg to 1000 µg were
administered orally, sublingually or rectally. Side-effects, primarily high fever
and shivering, were associated with higher doses; few life-threatening events
have been reported. Hence, doses of 1000–1200 µg are not recommended. The
Consultation noted that the largest trial of misoprostol for treatment of PPH
(35) reported use of a dose of 800 µg, given sublingually. The majority of the
participants felt that, in the treatment of PPH, where the first- and secondline uterotonics are not available or have failed, as a last resort 800 μg can be
used. However, three members strongly disagreed with this conclusion because
1
Final numbers were confirmed after the meeting by Gynuity.
of concerns about safety. Because of the disagreement the discussion of dose is
included here, rather than as a recommendation.
▪
In view of the uncertainty and disagreement among the participants regarding the
safe dose of misoprostol, WHO will commission a further review of misoprostol
doses and routes of administration.
(c) Should tranexamic acid be offered in the treatment of PPH due to
uterine atony?
Tranexamic acid is an antifibrinolytic agent that has been on the market for several
decades. Antifibrinolytic agents are widely used in surgery to reduce blood loss.
A systematic review of randomized controlled trials of antifibrinolytic agents in
elective surgery showed that tranexamic acid reduced the risk of blood transfusion
by 39% (36). Another Cochrane review showed that tranexamic acid reduced heavy
menstrual bleeding without side-effects (37).
Summary of evidence
There have been no RCTs on the use of tranexamic acid for the treatment of PPH
following vaginal delivery that address the priority outcomes. Tranexamic acid has
been evaluated as prophylaxis following caesarean section in one RCT (38). The
average blood loss in the two hours after the caesarean section was 42.75±40.45 ml
in the study group and 73.98±77.09 ml in the control group.
One case report was found of a woman given tranexamic acid for treatment of
massive postpartum haemorrhage after caesarean section (39).
Recommendation
Tranexamic acid may be offered as a treatment for PPH if: (i) administration of
oxytocin, followed by second-line treatment options and prostaglandins, has failed to
stop the bleeding; or (ii) it is thought that the bleeding may be partly due to trauma.
(Quality of evidence: very low. Strength of recommendation: weak.)
Remarks
Evidence for this recommendation was extrapolated from the literature on surgery
and trauma, which shows tranexamic acid to be a safe option in trauma-related
bleeding.
The benefits of use of tranexamic acid in PPH treatment should be investigated in
research studies.
(d) Should recombinant factor VIIa be offered in the treatment of
PPH due to uterine atony?
Recently, recombinant factor VIIa (rFVIIa) has generated interest as an option for
treatment of PPH, mainly in industrialized countries. The evidence regarding its use
in the treatment of PPH is limited to reviews of case reports and case series (40, 41)
and two observational studies (42,43) (page 30, GRADE Table B3).
Hossain (43) described a retrospective cohort study of 34 patients with more than
1500 ml blood loss in which 18 were treated with rFVIIa. Ahonen (42) compared the
outcomes of 26 women who received rFVIIa to those of 22 women treated in the
same time period for PPH without rFVIIa.
Both studies included women who had had caesarean section as well as women who
had had a vaginal birth. The causes of PPH included uterine atony as well as
abnormal placentation, retained placenta, and cervical or vaginal lacerations. The
women had received conventional treatments, such as uterotonics, uterine massage,
arterial ligation and, in some cases, hysterectomy prior to the administration of
rFVIIa.
The risk of maternal death appeared to be lower in women treated with rFVIIa (OR
0.38, 95%CI 0.09–1.60), and remained lower following adjustment for baseline
haemoglobin and activated partial thromboplastin time (aPTT) (OR 0.04, 95%CI 0.002–
0.83) (43). The risk of subsequent need for hysterectomy is difficult to ascertain,
as the drug was administered as a ‘last resort’ treatment. The authors note that as
confidence in its use increased, rFVIIa began to be offered prior to hysterectomy. In
Ahonen’s report (42), 8 women received rFVIIa following hysterectomy, but none of
the remaining 18 women treated with rFVIIa subsequently underwent hysterectomy.
A high rate of thrombotic events (185 events in 165 treated patients) has been
reported in patients receiving rFVIIa for off-label use (44). Ahonen (42) described
one report of a pulmonary embolus; the woman was subsequently diagnosed with
antithrombin deficiency.
The Consultation discussed the evidence from observational studies and heard about
ongoing research on rFVIIa.
Recommendation
The Consultation agreed that there was not enough evidence to make any
recommendation regarding the use of recombinant factor VIIa for the treatment of
PPH. Recombinant factor VIIa for the treatment of PPH should be limited to women
with specific haematological indications.
Remark
Use of rFVIIa could be life-saving, but it is also associated with life-threatening sideeffects. Moreover, recombinant factor VIIa is expensive to buy and may be difficult to
administer.
2. Non-medical interventions for management of PPH
A range of mechanical interventions to compress or stretch the uterus have been
proposed, either as temporizing measures or as definitive treatment. These
interventions are summarized below.
(a) Should uterine massage be offered in the treatment of PPH?
Uterine massage as a therapeutic measure is defined as rubbing of the uterus
manually over the abdomen sustained until bleeding stops or the uterus contracts.
Initial rubbing of the uterus and expression of blood clots is not regarded as
therapeutic uterine massage.
Summary of evidence
There have been no RCTs on the use of uterine massage in the treatment of PPH. A
case report series (45) and indirect evidence from one systematic review (46) on the
use of uterine massage in PPH prevention were found.
In one RCT of the prophylactic use of uterine massage involving 200 women,
massage was associated with a nonsignificant decrease in incidence of blood loss
>500 ml (RR 0.52, 95%CI 0.16–1.67) and a significant reduction in the use of additional
uterotonics (RR 0.20, 95%CI 0.08–0.50) (page 31, GRADE Table B4).
Recommendation
Uterine massage should be started once PPH has been diagnosed. (Quality of
evidence: very low. Strength of recommendation: strong.)
Remarks
▪
Uterine massage to ensure the uterus is contracted and there is no bleeding is a
component of active management of the third stage of labour for the prevention
of PPH.
▪
The low cost and safety of uterine massage were taken into account in making this
recommendation strong.
(b) Should bimanual uterine compression be offered in the treatment
of PPH?
Summary of evidence
No RCTs on the use of bimanual uterine compression in the treatment of PPH were
identified. One case report (47) was found.
Recommendation
Bimanual uterine compression may be offered as a temporizing measure in the
treatment of PPH due to uterine atony after vaginal delivery. (Quality of evidence:
very low. Strength of recommendation: weak.)
Remark
The Consultation noted that health care workers would need to be appropriately
trained in the application of bimanual uterine compression and that the procedure
may be painful.
(c) Should uterine packing be offered in the treatment of PPH?
Summary of evidence
No RCTs on the use of uterine packing in the treatment of PPH were found. Seven
case series and one case report (48–55), with a total of 89 women (the largest
involved 33 women), and four overviews were identified. Success rates (i.e. no need
for hysterectomy or other invasive procedure) ranging from 75% to 100% are reported
in these studies.
Recommendation
Uterine packing is not recommended for the treatment of PPH due to uterine
atony after vaginal delivery. (Quality of evidence: very low. Strength of
recommendation: weak.)
Remark
The Consultation noted that there was no evidence of benefit of uterine packing and
placed a high value on concerns regarding its potential harm.
(d) Should intrauterine balloon or condom tamponade be offered in
the treatment of PPH?
Summary of evidence
There have been no RCTs on the use of uterine tamponade in the treatment of
PPH. Nine case series and twelve case reports, evaluating 97 women (56–76), and
two reviews were identified (77, 78). The instruments used included SengstakenBlakemore and Foley catheters, Bakri and Rusch balloons, and condoms.
Case series have reported success rates (i.e. no need for hysterectomy or other
invasive procedure) ranging from 71% to 100%.
Recommendation
In women who have not responded to treatment with uterotonics, or if uterotonics
are not available, intrauterine balloon or condom tamponade may be offered in
the treatment of PPH due to uterine atony. (Quality of evidence: low. Strength of
recommendation: weak.)
Remark
The Consultation noted that the application of this intervention requires training
and that there are risks associated with the procedure, such as infection. The use
of uterine balloon or condom tamponade in the treatment of PPH was considered a
research priority.
(e) Should external aortic compression be offered in the treatment
of PPH?
Summary of evidence
No trials were found describing the use of external aortic compression in the
treatment of PPH. A prospective study was performed in Australia to determine the
haemodynamic effects of external aortic compression in nonbleeding postpartum
women (79). Successful aortic compression, as documented by absent femoral pulse
and unrecordable blood pressure in a lower limb, was achieved in 11 of 20 subjects.
The authors concluded that the procedure is safe in healthy subjects and may be
of benefit as a temporizing measure in treatment of PPH while resuscitation and
management plans are made. Subsequently, one case report from Australia described
the use of internal aortic compression as a temporizing measure to control severe
PPH due to placenta percreta at the time of caesarean section (80).
Recommendation
External aortic compression for the treatment of PPH due to uterine atony after
vaginal delivery may be offered as a temporizing measure until appropriate care is
available. (Quality of evidence: very low. Strength of recommendation: weak.)
Remarks
▪
External aortic compression has long been recommended as a potential life-saving
technique, and mechanical compression of the aorta, if successful, slows down
blood loss.
▪
The Consultation placed a high value on the procedure as a temporizing measure
in treatment of PPH.
(f) Should nonpneumatic antishock garments be offered in the
treatment of PPH?
Summary of evidence
There have been no RCTs on the use of pneumatic or nonpneumatic antishock
garments in the treatment of PPH. Case studies and case series have, however, been
published and summarized (81–87). The use of nonpneumatic antishock garments
(NASGs) has been reported in a before-and-after study of 634 women with obstetric
haemorrhage (43% with uterine atony) in Egypt (88).
Women treated with an NASG had a median blood loss 200 ml lower (range
300–100 ml lower) than women who received standard treatment (hydration with
intravenous fluids, transfusion, uterotonics, vaginal or abdominal surgery, as needed)
in the “before” period (i.e. before the introduction of NASG). The risk of blood
transfusion was higher in those treated with NASG (RR 1.23, 95%CI 1.06–1.43); the
risk of surgical procedures was not statistically significantly different (RR 1.35, 95%CI
0.90–2.02) (page 31, GRADE Table B5).
A cluster RCT (Miller S, personal communication) is under way in Zambia and
Zimbabwe to examine whether early application of an NASG by midwives prior to
transfer to a referral hospital can decrease morbidity and mortality. No data were
available for review.
Recommendation
The Consultation decided not to make a recommendation on this question.
Remark
The Consultation noted that research was ongoing to evaluate the potential benefits
and harms of this intervention, and decided not to make a recommendation until
these research results become available.
(g) Should uterine artery embolization be offered in the treatment
of PPH?
Percutaneous transcatheter arterial embolization of the uterine artery has been
reported from institutions that have adequate radiological facilities for this
intervention.
Summary of evidence
There have been no RCTs on the use of arterial embolization in the treatment of
PPH. One retrospective cohort study (89) compared 15 women treated with
embolization with 14 women receiving other treatments for PPH. The majority of
these patients had been transferred from local hospitals. Ten of 13 women were
successfully treated for PPH with arterial embolization. Of 11 women originally
treated with conservative surgical methods, two subsequently underwent arterial
embolization; one of these was successful while the second patient eventually
required hysterectomy. Eighteen case series and 15 case reports (90–122) have been
published, describing the intervention in 340 women. Studies report success rates
(i.e. no need for hysterectomy or other invasive procedures) ranging from 82% to
100% (page 32, GRADE Table B6).
Recommendation
If other measures have failed and resources are available, uterine artery embolization
may be offered as a treatment for PPH due to uterine atony. (Quality of evidence:
very low. Strength of recommendation: weak.)
Remark
Uterine artery embolization requires significant resources, in terms of cost of
treatment, facilities and training of health care workers.
3. Surgical interventions in the treatment of PPH
A wide range of surgical interventions have been reported to control postpartum
haemorrhage that is unresponsive to medical or mechanical interventions. They
include various forms of compression sutures, ligation of the uterine, ovarian or
internal iliac artery, and subtotal or total hysterectomy.
Summary of evidence
There have been no RCTs on the use of uterine compressive sutures in the treatment
of PPH. Thirteen case series and twelve case reports describing a total of 113 women
were identified (123–147). Eight overviews on compression sutures have also been
published (77, 78, 148–153). The B-Lynch technique seems to be the most commonly
reported procedure. Success rates (i.e. no need for hysterectomy or other invasive
procedure) range from 89% to 100%.
Similarly, no RCTs on the use of selective artery ligation in treatment of PPH
were identified. Twenty-one case series and 13 case reports have been published,
describing the intervention in 532 women (123, 154–186). Studies report success
rates (i.e. no need for hysterectomy or other invasive procedure) ranging from
62% to 100%.
Recommendation
If bleeding does not stop in spite of treatment with uterotonics, other conservative
interventions (e.g. uterine massage), and external or internal pressure on the uterus,
surgical interventions should be initiated. Conservative approaches should be tried
first, followed – if these do not work – by more invasive procedures. For example,
compression sutures may be attempted first and, if that intervention fails, uterine,
utero-ovarian and hypogastric vessel ligation may be tried. If life-threatening
bleeding continues even after ligation, subtotal (also called supracervical or total
hysterectomy) should be performed. (Quality of evidence: no formal scientific
evidence of benefit or harm. Strength of recommendation: strong.)
Remark
The Consultation acknowledged that the level of skill of the health care providers will
play a role in the selection and sequence of the surgical interventions.
C. Management of retained placenta
1. Should uterotonics be offered as treatment for retained placenta?
Summary of evidence
One double-blind RCT was found that compared sulprostone with placebo in
50 women with retained placenta (187). Originally designed to recruit over 100
patients, the trial was stopped prematurely and sulprostone was given to all
remaining cases.
The authors reported a lower risk of manual removal of the placenta (RR 0.51, 95%CI
0.34–0.86) and a similar risk of blood transfusion in the two groups (RR 0.81, 95%CI
0.33–2.00) (page 33, GRADE Table C1). There is no empirical evidence for or against
the use of other uterotonics for treatment of retained placenta.
Recommendations
▪
If the placenta is not expelled spontaneously, clinicians may offer 10 IU of
oxytocin in combination with controlled cord traction. (No formal scientific
evidence of benefit or harm. Strength of recommendation: weak.)
▪
Ergometrine is not recommended, as it may cause tetanic uterine contractions,
which may delay expulsion of the placenta. (Quality of evidence: very low.
Strength of recommendation: weak.)
▪
The use of prostaglandin E2 (dinoprostone or sulprostone) is not recommended.
(Quality of evidence: very low. Strength of recommendation: strong.)
Remarks
▪
The Consultation found no empirical evidence to support recommendation
of uterotonics for the management of retained placenta in the absence of
haemorrhage. The above recommendation was reached by consensus.
▪
The WHO guide, Managing complications in pregnancy and childbirth (18), states
that if the placenta is not expelled within 30 minutes after delivery of the baby,
the woman should be diagnosed as having retained placenta. Since there is no
evidence for or against this definition, the delay used to diagnose this condition is
left to the judgement of the clinician.
▪
The same guide also recommends that, in the absence of haemorrhage, the
woman should be observed for a further 30 minutes following the initial
30 minutes, before manual removal of the placenta is attempted. The
Consultation noted that, in the absence of bleeding, spontaneous expulsion of the
placenta can still occur; thus, a conservative approach is advised and the timing
of manual removal as the definitive treatment is left to the judgement of the
clinician.
▪
The recommendation about prostaglandin E2 is based on the lack of evidence, as
well as concerns regarding adverse events, notably cardiac events.
2. Should intra-umbilical vein injection of oxytocin with or without
saline be offered as treatment for retained placenta?
Summary of evidence
One systematic review on umbilical vein injection for the management of retained
placenta has been published (188). RCTs comparing the use of intraumbilical
vein injection of saline with expectant management (four studies, 413 women),
intraumbilical vein injection of saline+oxytocin with expectant management
(five studies, 454 women), and intraumbilical vein injection of saline+oxytocin with
saline (ten studies, 649 women) were identified.
The results of one unpublished study (189) were made available to the Consultation
by the investigators. In this multicentre trial, 577 women in Pakistan, Uganda and the
United Kingdom were randomized to receive either intraumbilical vein injection of
50 IU of oxytocin in 30 ml of saline (n=292) or matching placebo (n=285).
Intraumbilical vein injection of saline versus expectant management
There were no significant differences in rates of manual removal of the placenta
(RR 0.97, 95%CI 0.83–1.19), blood loss ≥500 ml (RR 1.04, 95%CI 0.55–1.96), blood loss
≥1000 ml (RR 0.73, 95%CI 0.17–3.11), or blood transfusion (RR 0.76, 95%CI 0.41–1.39)
(page 34, GRADE Table C2).
Intraumbilical vein injection of saline+oxytocin versus expectant management
There was a slightly lower rate of manual removal of the placenta in the group given
saline+oxytocin, although the difference was not statistically significant (RR 0.86,
95%CI 0.72–1.01). Rates of blood loss ≥500 ml (RR 1.53, 95%CI 0.78–2.67), blood loss
≥1000 ml (RR 1.29, 95%CI 0.38–4.34), and blood transfusion (RR 0.89, 95%CI 0.5–1.58)
were similar with wide confidence intervals (page 35, GRADE Table C3).
Intraumbilical vein injection of saline+oxytocin versus saline
There was a lower risk of manual removal of the placenta in the group given
saline+oxytocin (RR 0.79, 95%CI 0.69–0.91). No differences were found in rates of
blood loss ≥500 ml (RR 1.43, 95%CI 0.83–2.45), blood loss ≥1000 ml (RR 1.71, 95%CI
0.45–6.56) or blood transfusion (RR 1.17, 95%CI 0.63–2.19) and the confidence intervals
were wide because there were few events (page 36, GRADE Table C4).
The unpublished RELEASE trial (189) data showed no benefit of intraumbilical vein
injection of saline with oxytocin over placebo in terms of manual removal of the
placenta (RR 0.98, 95%CI 0.87–1.12), blood loss ≥500 ml (RR 0.98, 95%CI 0.78–1.23),
blood loss ≥1000 ml (RR 1.09, 95%CI 0.67–1.76) and blood transfusion (RR 0.77, 95%CI
0.46–1.26) (page 37, GRADE Table C5).
Recommendations
▪
Intraumbilical vein injection of oxytocin with saline may be offered for the
management of retained placenta. (Quality of evidence: moderate. Strength of
recommendation: weak.)
▪
If, in spite of controlled cord traction, administration of uterotonics and
intraumbilical vein injection of oxytocin+saline, the placenta is not delivered,
manual extraction of the placenta should be offered as the definitive treatment.
(No formal assessment of quality of evidence. Strength of recommendation:
strong.)
Remarks
▪
During the discussion on this topic, a new meta-analysis of the available data was
performed, by including data from the recent large unpublished study with the
existing published meta-analysis. Sensitivity analyses by quality of data and a
fixed-versus-random-effects analysis were also conducted. In all these secondary
analyses, the summary estimate reflected a modest effect, with the relative risk
of manual removal being 0.89 (95%CI 0.81–0.98) with a fixed-effect model and 0.82
(95%CI 0.68–0.98) with a random-effect model. The Consultation was concerned
about the possibility of publication bias in the meta-analysis, and was split
between making a weak recommendation and not recommending intra-umbilical
vein injection of oxytocin+saline.
▪
The Consultation recommended by a majority the use of umbilical vein injection
of oxytocin+saline for retained placenta. In making the recommendation, the
Consultation considered the advantages of avoiding an invasive intervention, such
as manual removal of the placenta, and the low cost and absence of any sideeffects with umbilical vein injection. It was noted that a potential disadvantage
was that this intervention may delay the administration of other effective
interventions. These considerations should be taken into account in the local
adaptation of these guidelines.
3. Should antibiotics be offered after manual extraction of the
placenta as part of the treatment of retained placenta?
Summary of evidence
A systematic review of antibiotic prophylaxis after manual removal of the placenta,
published in 2006, found no RCTs (190).
One retrospective study (191) of 550 patients evaluated prophylactic antibiotic
therapy in intrauterine manipulations (such as forceps delivery, manual removal of
the placenta and exploration of the cavity of the uterus) during vaginal delivery.
Recommendation
A single dose of antibiotics (ampicillin or first-generation cephalosporin) should be
offered after manual removal of the placenta. (Quality of evidence: very low.
Strength of recommendation: strong.)
Remarks
▪
Direct evidence of the value of antibiotic prophylaxis after manual removal of the
placenta was not available. The Consultation considered indirect evidence of the
benefit of prophylactic antibiotics from studies of caesarean section (192) and
abortion, and observational studies of other intrauterine manipulations.
▪
Current practice suggests that ampicillin or first-generation cephalosporin may be
administered when manual removal of the placenta is performed.
▪
This question was identified as a research priority for settings in which
prophylactic antibiotics are not routinely administered and those with low
infectious morbidity.
D. Choice of fluid for replacement or resuscitation
1. Should crystalloids be offered for fluid replacement in women
with PPH?
Fluid replacement is an important component of resuscitation for women with PPH,
but the choice of fluid is controversial. Although outside the initial scope of these
guidelines, this question was put to the Consultation in view of its importance.
Summary of evidence
There have been no RCTs comparing the use of colloids with other replacement fluids
for resuscitation of women with PPH. There is indirect evidence from a Cochrane
review that evaluated 63 trials on the use of colloids in the resuscitation of critically
ill patients who required volume replacement secondary to trauma, burns, surgery,
sepsis and other critical conditions (193). A total of 55 trials reported data on
mortality for the following comparisons.
Colloids versus crystalloids
No statistical difference in the incidence of mortality was found when albumin or
plasma protein fraction (23 trials, 7754 patients, RR 1.01, 95%CI 0.92–1.10),
hydroxyethyl starch (16 trials, 637 patients, RR 1.05, 95%CI 0.63–1.75), modified
gelatin (11 trials, 506 patients, RR 0.91, 95%CI 0.49–1.72), or dextran (nine trials, 834
patients, RR 1.24, 95%CI 0.94–1.65) were compared with crystalloids (page 38, GRADE
Table D1).
Colloid versus hypertonic crystalloid
One trial, which compared albumin or plasma protein fraction with hypertonic
crystalloid, reported one death in the colloid group (RR 7.00, 95%CI 0.39–126.92).
Two trials that compared hydroxyethyl starch and modified gelatin with crystalloids
observed no deaths among the 16 and 20 participants, respectively (page 39,
GRADE Table D2).
Colloids in hypertonic crystalloid versus isotonic crystalloid
The outcome of death was reported in eight trials, including 1283 patients, which
compared dextran in hypertonic crystalloid with isotonic crystalloid (RR 0.88, 95%CI
0.74–1.05) and in one trial with 14 patients (page 39, GRADE Table D3).
Recommendation
Intravenous fluid replacement with isotonic crystalloids should be used in preference
to colloids for resuscitation of women with PPH. (Quality of evidence: low. Strength
of recommendation: strong.)
Remark
Available evidence suggests that high doses of colloids, which are more expensive
than isotonic crystalloids, may cause adverse effects.
E. Health systems and organizational interventions
1. Should health care facilities have a protocol for management of PPH?
Summary of evidence
The literature search did not reveal any research evidence for or against the use
of PPH management protocols. Although no systematic review was carried out, the
Consultation considered that management protocols are generally useful and unlikely
to be harmful.
Recommendation
Health care facilities should adopt a formal protocol for the management of PPH.
(Quality of evidence: no formal evidence reviewed; consensus. Strength: strong.)
Remark
The Consultation acknowledged that the implementation of formal protocols is a
complex process, which will require local adaptation of general guidelines.
2. Should health care facilities have a formal protocol for referral of
women diagnosed as having PPH?
Summary of evidence
The literature search did not reveal any research evidence for or against the use of
PPH referral protocols. Although no systematic review was carried out, the
Consultation considered that referral protocols are generally useful and unlikely to be
harmful.
Recommendation
Health care facilities should adopt a formal protocol for patient referral to a higher
level of care. (Quality of evidence: no formal evidence reviewed; consensus.
Strength of recommendation: strong.)
3. Should simulation of PPH treatment be part of
training programmes for health care providers?
Summary of evidence
The literature search did not reveal any research evidence for or against
the use of PPH simulation programmes. Although no systematic review was
carried out, the Consultation considered that PPH simulation programmes
are generally useful and unlikely to be harmful.
Recommendation
Simulations of PPH treatment may be included in pre-service and in-service
training programmes. (Quality of evidence: no formal evidence
reviewed; consensus. Strength of recommendation: weak.)
Remarks
This recommendation is extrapolated from non-obstetric literature.
The Consultation placed a high value on the costs of simulation
programmes acknowledging that there are different types of simulation
programmes. Some of those programmes are hi-tech, computerized
and costly while others are less expensive and more likely to be
affordable in low and middle income countries.
The Consultation identified improvement in communication between health
care providers and patients and their family members as an important
priority in training of health care providers in PPH management.
The Consultation identified this area as a research priority.
PPH care pathways
Postpartum haemorrhage can present in different clinical scenarios. Bleeding may
be immediate and in large amounts, it may be slow and unresponsive to
treatments, or it may be associated with systemic problems, such as clotting
disorders. The recommendations related to PPH prevention, namely, active
management of the third stage of labour, should be routinely applied (1).
It is critical that health workers remain vigilant during the minutes and
hours following birth, in order to identify problems quickly. The care
pathways presented (see insert) assume the presence of a skilled caregiver
and a facility with basic surgical capacity. A stepwise approach is
recommended. The initial step is to
assess the woman and take immediate nonspecific life-saving measures,
such as resuscitation, calling for help and monitoring vital signs. The
second step is to give directive therapy following the diagnosis of PPH. In
a given clinical situation, not
all diagnostic assessments can be done simultaneously. The caregiver
should assess the situation according to the circumstances surrounding the
birth and immediate subsequent events.
First trimester abortion
The duration of pregnancy (gestation duration) is conventionally divided into three
trimesters: first trimester, up to 14 weeks of gestation since the first day of the
last menstrual period; second trimester, 14–28 weeks; third trimester, 28 weeks to
delivery (at 40 weeks, on average).4 First trimester surgical abortion (up to 12 or 14
weeks) and medical abortion (up to nine weeks) should be performed as outpatient
procedures.
Pre-abortion visit
Clinical aspects
Counselling
History taking
As outlined in the Introduction, counselling must be
Take a note of the following:
supportive and empathetic. If the client is accompanied,
• The woman’s age.
make sure that some counselling time is set aside for the
• Her marital status (if necessary; not mandatory).
client alone. Involve the accompanying person later and also
• Previous pregnancies (children and their health,
provide information to him or her on caring for the client.
If the woman agrees, explore the following issues:
• Is she seeking to continue the pregnancy, to have the
baby adopted or to have an abortion?
• What support is available if she decides to continue the
pregnancy or to opt for adoption?
• Is she being coerced into seeking an abortion?
• The legal aspects of abortion, and the need to sign an
informed consent form.
• Her reasons for seeking an abortion, including her
miscarriage(s), ectopic pregnancy, induced abortions),
as well as all types of complications during previous
pregnancies.
• Duration of current pregnancy (since the first day of last
menstrual period). Confirm with clinical examination.
See General physical examination on page 7 and
Gynaecological examination on page 7.
• Any attempt to abort current pregnancy (and details, if
relevant).
• Past and present medical and surgical (especially
socio-cultural and economic circumstances. This is,
abdominal) conditions that might affect an abortion
however, not mandatory, so only explore these reasons
procedure, for example a bleeding disorder, reproductive
if the discussion goes in this direction. Many women do
tract infections, allergies (to anaesthetics, antibiotics
not want to address these issues, and their wishes must
or other), medication taken, and contraception used,
be respected. If there are indications of violence, be ready
correctly or otherwise.
to provide referrals for further support.
• Levels of anxiety and guilt.
• Support (or absence of support) from partner/friends/
family.
• The possibility of sexual abuse or gender-based violence.
• The various options (if relevant) of abortion techniques
and pain control.
• Previous female genital mutilation: this could complicate
the procedures.
• Past experience with contraceptive methods and future
contraception being considered.
• If the pregnancy is not established, ask if symptoms
of early pregnancy are present: breast tenderness and
engorgement, nausea, fatigue, frequent urination and/or
changes in appetite.
4
Subtract two weeks from these timings if calculating from the day of presumed or proven ovulation.
Chapter 2 First trimester abortion
Clinical assessment
• A uterus bigger than expected suggests multiple
a) General physical examination
pregnancy (polycyesis: more than one foetus), molar
Check or examine:
pregnancy or fibroids.
• General health (pulse, blood pressure, heart, lungs and
• A uterus smaller than expected suggests a miscarriage, no
temperature). Check for anaemia, malnutrition and other
pregnancy or ectopic pregnancy. If pregnancy is suspected
signs of ill health. Refer if necessary to a higher level
and the uterus is still small six weeks after the last
provider for a pre-abortion medical assessment.
menstrual period, use ultrasound for checking, or hCG,
• Abdomen (ask the woman to empty her bladder
beforehand). Is it distended? Are there masses? Can the
uterus be felt? If so, assess its size.
or refer. In the case of ectopic pregnancy, take immediate
action by medical or surgical treatment, applying local
guidelines, or refer immediately.
• Determine the position of the uterus (ante- or retroflexed;
b) Gynaecological examination
Pelvic examination and speculum
ante- or retroverted).
• Check for reproductive tract infections (uterine,
• Ask the woman to empty her bladder beforehand.
tubo-ovarian), sexually transmitted infections and cystitis.
• Ensure privacy and, where customary, the presence of a
If antibiotics are indicated, start immediately,
female chaperone if the provider is male.
• Ensure adequate light.
• Speak kindly to the woman; explain the procedures and
BEFORE the abortion, but do not delay the abortion
once the antibiotics have been started.
• Record all the findings in the client’s file.
help her relax.
• Wash hands and use sterile or disinfected gloves; proceed
gently.
• Inspect the perineal area, and check for signs of sexually
transmitted infections: ulcers, condyloma, discharge.
c) Special investigations
Ultrasound and laboratory testing are special procedures, but
their availability should not be a prerequisite for the provision
of abortion services. See Annex 1 for details.
Check for vulvitis, female genital mutilation and scars.
Swab the vulva. Treat as needed.
• Gently insert a closed speculum, of the smallest effective
Prophylactic (preventive) antibiotics
Most, but not all protocols for comprehensive abortion
size, moistened with warm water (gels and lubricants
care recommend the routine administration of prophylactic
interfere with diagnostic accuracy for sexually transmitted
antibiotics. Follow local guidelines. World Health
infections). Avoid pressure on the urethra. Check for,
Organization5 and IPPF guidance6 state that this reduces
diagnose and treat vaginal and cervical infections,
the post-procedural risk of infection, but that abortion
according to local protocols.
should not be denied where prophylactic antibiotics are not
• If indicated and available, perform cervical cytology or
available.
inspection (not a precondition to provide the abortion).
Counselling: contraception, follow-up, questions and answers
Bimanual pelvic examination
a) Contraception
• Wash hands and use sterile or disinfected gloves; proceed
A wide choice of contraceptive methods should be made
gently.
available, including sterilization (if relevant, although hasty
• Confirm pregnancy (soft cervix; enlarged, soft uterus).
decisions should not be made). Ensure that contraceptive
• Check that the size of the uterus corresponds to the
information relating to the available options is made
gestation duration. A discrepancy may be due to an
available before the procedure, before leaving the clinic
error in the calculation, or indicate a problem with the
on the day of the procedure, and during subsequent
pregnancy.
post-abortion visit(s), but not during the procedure itself
when the woman is under emotional or physical stress.
5
World Health Organization (2003) Safe Abortion: Technical and Policy Guidance for Health Systems, www.who.int/reproductive-health/publications/
safe_abortion/safe_abortion.pdf
6
International Planned Parenthood Federation (2004) IPPF Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services,
www.ippf.org
7
Chapter 2 First trimester abortion
Acceptance of contraception or of a particular method must
Surgical or medical abortion?
never be a prerequisite for the provision of abortion-related
First trimester abortion can be provided using either medical
services. See Chapter 5 for information about post-abortion
drug-induced abortion, up to nine weeks, or surgical
contraception.
techniques. Both can be provided by trained mid-level staff
(nurses, midwives and medical assistants, according to the
b) Explaining the procedure and the follow-up
defined scopes of practice at the national level). Table 1
Address the following issues: how long the procedure will
summarizes the advantages and disadvantages of the two
take; the possible side-effects (for example pain, bleeding,
approaches. If both options are suitable, the patient’s choice
nausea, diarrhoea, vomiting); the recovery time; possible
should be respected.
complications and where to go for treatment; safety aspects
Surgical abortion can be performed by aspiration (using
and the risks (all medical interventions carry some risks); the
an electric pump or a manual syringe) or by dilatation and
choice of anaesthesia and options for pain management;
curettage (sometimes called dilation and curettage or D&C).
and when to resume normal activities (including sexual
Dilatation and curettage is an outdated surgical technique
intercourse). Stress the need for contraception and condom
that should be replaced, whenever possible, by aspiration or
use, the need for follow-up and explain the content of the
medical (drug-induced) abortion which are better options, as
follow-up visit.
recommended by the World Health Organization and IPPF.7
A surgical technique (preferably aspiration) should be
c) Questions and answers
used if:
Allow sufficient time for the client to ask questions and
• it is the woman’s choice
express any fears.
• concurrent sterilization is anticipated
• there are contraindications to medical abortion
• a client is unable to come for follow-up after a medical
abortion as required
Table 1: Advantages and disadvantages of medical and surgical abortion
Advantages
Medical abortion
Surgical abortion
• Avoids surgery and anaesthesia
• Quicker
• More ‘natural’, like menses
• More likely to have a complete abortion
• Emotionally easier for some women
• Emotionally easier for some women
• Client controlled; more privacy and autonomy; can be
home-based
• Better than surgical in very early gestation, or with
• Takes place in a health care centre, clinic or hospital
• Can be used up to 14 weeks (12–14 weeks by experts only)
• Sterilization can be concurrent 8
severe obesity (body mass index >30) without other
cardiovascular risk factors, or in the case of fibroids,
uterine malformations or previous cervical surgery
• No risk of cervical/uterine injury
Disadvantages • Bleeding, cramping, nausea, diarrhoea and other
side-effects
• Invasive
• Small risk of cervical or uterine injury
• Waiting, uncertainty
• Risk of infection
• More clinic visits
• Less privacy and autonomy
• Drugs are costly
• Can be costly
• Can only be used up to nine weeks
7
See notes 5 and 6 on page 7.
8
Concurrent sterilization should never be done without the patient’s informed consent, and never hastily without proper thought beforehand.
7
Chapter 2 First trimester abortion
Surgical abortion
epinephrine) beneath the cervical mucosa at 2, 4, 8 and
Preparation
10 o’clock around the cervix (be careful not to inject into a
• Ensure that the consent form has been signed.
blood vessel). The World Health Organization provides a full
• Ensure that the equipment is in working order.
description of the procedure.9
• Double check that the resuscitation equipment is in
working order.
Signs of a toxic reaction and its treatment are described
in Table 2.
Light (or conscious) sedation: This can replace the
Pain control (and its possible complications)
paracervical block. The procedure alters a woman’s state
Pain may result from cervix dilatation, internal cervical
of consciousness, without the risks associated with general
os stimulation, uterus mobilization, scraping the uterine
anaesthesia, helping her to feel more relaxed and less
walls and reactive uterine muscular contractions. Intense
anxious during the abortion. The patient breathes normally
pain can result from cervical dilatation that is too forceful
and is easily roused. She can respond to physical stimuli and
or from intervention too soon after paracervical block.
verbal commands.
Pain thresholds vary; and fear and anxiety can augment
pain. Providers should be calm, efficient, friendly and
The management of anaesthesia-related adverse effects
and possible complications is presented in Table 2.
non-judgemental, and explain what is happening (or what
General anaesthesia is not recommended because
the client should expect to experience) all the way through
narcosis adds risks. It can, however, be used on demand
the procedure. Communication with the client should be
or in abortion procedures that are difficult, medically or
maintained at all times.
emotionally. Skilled staff and the capacity to manage
Supportive verbal communication is also required during
complications are essential.
the procedure. Where possible, and preferably, a supportive
person should accompany the patient throughout the
Cervical dilatation (or ‘cervical priming’)
procedure. Counselling, empathy, ‘verbal anaesthesia’ and
• Cervical priming is a pre-intervention dilatation of the
fear reduction can all help, but pain control measures must
cervix, making aspiration (or dilatation and curettage)
always be available and provided. The following methods
easier and quicker, and reducing the risk of cervical
can be used singly or in combination. It should be noted
laceration and, to a lesser degree, the risk of uterine
that even if pain-relieving methods are used, including verbal
perforation.
anaesthesia, it is impossible to suppress pain totally.
Analgesia: Analgesics such as paracetamol
• Routine cervical preparation remains controversial because
the advantages have been insufficiently proven. Some
(acetaminophen) and ibuprofen ease pain. Opiates should
providers use priming only in specific circumstances,
be used with caution as post-abortion incidents have been
such as late first trimester abortions at 12–14 weeks of
described, such as falls on stairs and road accidents. To avoid
pregnancy, adolescents and young women, nulliparity,
this, the patient should be accompanied by someone who
scarred or diseased cervix, perceived risk of cervical injury
can try to avoid any problems and deal with any incident
that may arise.
Tranquillizers (anxiolytics): These reduce anxiety and
memory, but not pain, and relax the muscles. When using
benzodiazepines, such as diazepam, the same advice as for
opiates (see paragraph above) should be followed.
Paracervical block: This is always required for surgical
or uterine perforation, or inexperienced provider.
• Dilatation and curettage can sometimes be performed
without any cervical dilatation, if it is very easy to pass the
cannula through the inner cervical os.
• Cervical priming is done using laminaria tents
(Dilapan-S™ and Lamicel®, osmotic hydrophilic dilators)
introduced into the cervical canal, or a prostaglandin
abortion, unless the patient formally refuses it. Use a local
(for example misoprostol) or mifepristone. Compared to
anaesthetic such as lidocaine (lignocaine or xylocaine).
laminaria, vaginal misoprostol acts faster, is associated
Ensure there is no known allergy to lidocaine. Inject four
with less discomfort and is preferred by patients.
doses of 5mL (total 20mL) of 0.5 per cent lidocaine (without
Sublingual misoprostol (400µg), at least two hours before
9
World Health Organization (2003) Managing Complications in Pregnancy and Childbirth: A Guide for Midwives and Doctors,
www.who.int/reproductive-health/impac/Procedures/Paracervical_block_P1_P2.html
9
Chapter 2 First trimester abortion
Table 2: Pain control in first trimester induced abortion: possible adverse effects and their treatment
Type of
intervention
Verbal
anaesthesia
Measures
Possible adverse effects, complications and management
• Throughout the procedure, explain each step in simple
• Some patients prefer silence
language before it is performed
• Medical assistant talks softly and reassuringly to
patient
• Avoid saying “this won’t hurt” or “it’s almost done”
(when it is not)
• Do not restrict pain medication
• Ask patient to breathe slowly in through her nose
and out through her mouth to help her focus more on
breathing than on pain
• Have a relaxing picture painted on the ceiling, to focus
on; soft music can help (if the patient wants it)
Oral analgesia
• Use paracetamol 500–1,000mg or ibuprofen
• Do not use aspirin which increases bleeding
400–800mg
• Wait 30–60 minutes for it to take effect
• Tramadol 50mg, an opiate, should be avoided or used
with caution
Anxiolysis
• Diazepam 5–10mg orally
• Dizziness: after the procedure, the patient should be
accompanied at all times until the symptom wears off, and
refrain from using stairs, driving, handling machinery, walking
long distances
Local
anaesthesia:
paracervical
block with
lidocaine
• The provider must be trained to administer paracervical • Toxic reaction (rare): avoid by using the smallest effective
block
• Ensure there is no allergy to local anaesthesia
• Lidocaine 1 per cent without epinephrine, limited
to 3.5mg/kg body weight and, in any event, to a
maximum of 20mL
dose; aspirate before each injection
• Mild reaction (numbness of tongue and lips; metallic taste in
mouth; dizziness or light-headedness; ringing in ears, difficulty in
focusing eyes): wait and support verbally
• Severe reaction (sleepiness, slurred speech, disorientation,
• Aspirate before injecting lidocaine
muscle twitching, convulsions, loss of consciousness, respiratory
• Wait 4–5 minutes for it to take effect
depression): oxygen, diazepam 5mg IV, slowly; allergy (hives,
rash): diphenhydramine 25–50mg IV
• Respiratory distress: Ambu bag, oxygen, epinephrine 0.4mg
(1:1000 solution) SC
IV analgesia (by
specialist only)
• Fentanyl 0.05–0.06mg plus midazolam 0.5–1mg
• Narcotics (and IV diazepam) can depress or even halt respiration;
assist respiration with Ambu bag and oxygen. Reverse opiate
analgesics with naloxone 0.4mg IV (a second dose might be
necessary). Reverse diazepam with flumazenil 0.2mg IV
10
Chapter 2 First trimester abortion
the procedure, is effective. Oral administration is also
Manual vacuum aspiration procedure
effective, but higher doses and longer treatment periods
• Reassess the size, position (‘version’) and flexion of the
(8–12 hours) are required. Vaginal administration (400µg)
results in the same dilatation (mean: 8.5mm) as oral
administration with fewer side-effects (nausea, vomiting,
uterus by bimanual examination.
• Prepare two syringes (in case one is defective; discard if it
does not hold a vacuum).
diarrhoea and chills after vaginal misoprostol are 10,
• Prepare several cannulas according to uterine size (inspect
8, 18 and 4 times lower, respectively, than after oral
and discard if there are cracks or defects or any signs of
misoprostol). Oral mifepristone (600mg) is also effective
in cervical priming when administered 48 hours before
weakness or wear).
• Ask the patient, if conscious, to relax. Insert a comfortably
a first trimester abortion. However, the cost is high and
warm sterile speculum of the smallest adequate size into
availability low, in comparison with misoprostol.
the vagina.
• Misoprostol or mifepristone can be self-administered.
• Carefully spread the labia with a gloved hand; slowly
insert the speculum blades downward and inward. Slowly
Vacuum aspiration
open the blades, letting the cervix appear between them.
Vacuum aspiration is a very safe, effective procedure which
• Using a sponge forceps, wipe the cervix three times, in
should, whenever possible, replace dilatation and curettage.
a circular outward movement, using a compress with
It is used up to 12 weeks from last menstrual period. Expert
antiseptic.
providers can use it up to 14 weeks. Aspiration is more than
• Grasp the cervix with the tenaculum that has been placed
99.5 per cent effective. The incidence of haemorrhage, pelvic
for injecting the paracervical block, or place a forceps
infection, cervical injury and uterine perforation is lower than
at the 6 or 12 o’clock position. Straighten the uterus by
with dilatation and curettage, and less cervical dilatation is
exerting gentle traction on the tenaculum or the forceps
necessary. The costs of the procedure, the staff time and
resources needed are lower. No operating theatre or general
anaesthesia is needed.
placed on the cervix.
• Hysterometry: gently insert a uterine sound (hysterometer)
bent to the estimated angle of the uterine flexion, until
resistance is met. Read the depth of the uterine cavity by
Manual vacuum aspiration
noting the level of the mucous or blood on the sound.
Manual vacuum aspiration requires a single or double valve
Make a mental note of the depth of the uterine cavity.
syringe: the vacuum (at least 55mmHg) is generated by a
Note that according to most practitioners and
60mL hand-held syringe which accommodates flexible plastic
research results, this step is not only unnecessary but
cannulas ranging from 4mm to at least 12mm in diameter. A
may also be dangerous because it carries a risk of
chart explaining the use of the syringe can be downloaded
perforation.
from
Ipas.10
Infection prevention procedures and procedures for the
• Gently try to insert the cannula (without cervical dilatation
if the passage is very easy, which is usually the case up to
reuse of manual vacuum aspiration instruments can be
six weeks since last menstrual period). Carefully note the
downloaded from Pathfinder International.11
position and flexion of the uterus. Do not use force to
The first visit (see Pre-abortion visit on page 6), the pain
control techniques (see Pain control on page 9) and the
cervical priming techniques (see Cervical dilatation on
page 9) are the same as for dilatation and curettage and
are described earlier.
dilate the cervix.
• If there is resistance in the passage, use dilators to open
the cervix gradually.
• Once the cannula is inserted, use slow, gentle back and
forth movements, and do not use excessive force (to
avoid a perforation). Aspirate all tissues. There should
Electric vacuum aspiration
be no scraping of the uterine walls: the endometrium
This uses an electric pump. The technique is fundamentally
detaches under the influence of the negative pressure.
the same as with manual vacuum aspiration.
• When the uterus is empty, a strong uterine contraction
10 Ipas (2005) Tips for Using the Ipas MVA Plus, http://ipas.org/Library/Other/Tips_for_Using_the_Ipas_MVA_Plus.pdf
11 Pathfinder International (2000) Module 11: MVA for Treatment of Incomplete Abortion, pp73–99, www.pathfind.org/pf/pubs/module11.pdf
11
Chapter 2 First trimester abortion
Table 3: Uterine size and corresponding cannula size to be used for manual vacuum aspiration
Approximate uterine size
(expressed as corresponding gestation duration)
Approximate cannula size
5–6 weeks since day 1 of last menstrual period
5–6mm
7–8 weeks since day 1 of last menstrual period
7mm
9–10 weeks since day 1 of last menstrual period
7–10mm
10–12 weeks since day 1 of last menstrual period
9–12mm
can be felt as the uterus grips the cannula, making the
• After sedation or general anaesthesia, observe until
aspiration more difficult. Bubbles and red foam appear in
the patient’s full recovery. Discharge after anaesthetist’s
the cannula. The provider can detect a rough sensation in
authorization.
the uterus. These signs indicate that the uterus is empty
and that the procedure is complete. The last contents
of the aspirate may be only a few drops of pure blood.
When the uterus is empty, first stop aspirating, and only
then remove the cannula. No additional curettage or
• Record pain. Excessive pain could signal uterine
perforation or acute haematometra (blood filling the
uterus). See Table 4.
• Ensure the woman is able to pass urine before she is
discharged.
‘verification with curette’ is needed.
• The products of conception must be examined by the
Instructions to provide (verbal and written) and counselling
provider or medical assistant:
• How to use medications, if relevant.
– to ensure complete evacuation; if incomplete, repeat
• Some light bleeding or spotting may continue for several
the aspiration
weeks.
– to check for molar pregnancy (send for histology, if
• Normal menstruation should begin within 4–8 weeks.
• Nausea, and sometimes vomiting, can last for up to 24
necessary).
• In the absence of conception products, check for failure
of the procedure, duplicate uterus, perforation or ectopic
pregnancy. Routine laboratory examination of the
hours.
• Some cramps may occur over a few days. The patient
should take analgesics (paracetamol).
• The patient must know where to go if there is excessive
products is not essential.
bleeding, excessive pain, fever, fainting or other worrying
Early complications and their management
situations. An effective referral system must be in place.
The management of early complications needs trained staff,
The referral must be to a place that is accessible at all
adequate equipment and a functional referral system. Staff
times.
must be trained to re-evacuate the uterus.
A useful publication about complications after surgical
abortion is available from the World
Health Organization.12
• No douching and no tampons.
• Sex: not until it is desired by the woman. No sexual
intercourse until a few days after the main bleeding stops.
• Stress the need to use contraception as soon as sexual
Monitoring during the recovery period
• Check vital signs on the treatment table.
• No sedation or general anaesthesia: place the patient in a
comfortable position in the recovery room. Observe for 30
minutes.
activity is resumed.
• Make a formal appointment for a follow-up visit five
weeks later, or earlier if preferred.
• The client should be given the opportunity to ask
questions and seek any further support she may need.
12 World Health Organization (1994) Clinical Management of Abortion Complications: A Practical Guide,
www.who.int/reproductive-health/publications/clinical_mngt_abortion_complications/text.pdf
12
Chapter 2 First trimester abortion
Table 4: Early complications of surgically induced abortion (manual vacuum aspiration or dilatation and curettage) and their
management
Complication
Signs
Uterus is found empty
• Minimal material, or none,
collected
Verify
• No pregnancy? Test
• Ectopic pregnancy? Use
ultrasound if available
• Possible recent complete
spontaneous abortion
• Possible duplicate uterus: rare
Allergy to a drug
• Hives (itchy skin eruption,
• Check drug allergies
swollen red bumps or patches),
Treatment
• If (intrauterine) pregnancy is
confirmed, repeat aspiration
• Ectopic pregnancy is a lifethreatening complication.
Provide emergency local
treatment, or refer
• Administer diphenylhydramine
25–50mg IV
• Severe reaction: 0.3cc
rash
• Occasionally: difficulty breathing
epinephrine 1:1000 SC (can be
repeated once, and then every
15–20 minutes)
• Oxygen, start IV, protect airway
Toxic reaction to lidocaine
See Table 2
Incomplete evacuation
• Tissue obtained does not
Broken tip of plastic cannula
(rare)
• If there is any doubt this could
correspond to the pregnancy
be an indicator for using a
duration. Signs can include
curette. The procedure should be
vaginal bleeding and abdominal
performed smoothly, and only by
pain
trained staff
• Cannula is broken
• Repeat the procedure
• Do not keep exploring the cavity.
Use a fresh cannula to complete
the procedure. The detached tip
can either be aspirated or left (it
will be expelled spontaneously)
Perforation during hysterometry • The instrument penetrates
(a procedure NOT recommended)
beyond the expected size of the
uterus
• Usually resolves without any
need for surgical intervention.
Observe for 24 hours or refer.
Give ergometrine 0.2mg (add
one or maximum two doses if
bleeding is still present)
Suspected uterine or cervical
perforation with incomplete
evacuation
• Perforation during manual
vacuum aspiration is rare when
using flexible cannulas. Suspect
perforation if the cannula passes
• Stop the procedure
immediately and remove the
cannula
• Manage shock if necessary (see
through the uterine cavity
Table 7); do not administer
without any resistance or goes
methergine or oxytocin if there is
in much further than expected
suspected omentum or intestinal
given the uterine size, if it is
loop hernia
difficult to withdraw the cannula,
• Transfer the patient to an
if omental fat or pieces of small
obstetrics/gynaecology
bowel are found in the aspirate,
department at a hospital
or if there are signs of shock
13
Chapter 2 First trimester abortion
Table 4 continued: Early complications of surgically induced abortion (manual vacuum aspiration or dilatation and curettage) and their
management
Complication
Signs
Suspected uterine or cervical
• The cannula has penetrated
perforation after the evacuation
beyond the expected size of the
is complete
uterus
Verify
• Verify if uterus has been
Treatment
• Begin IV fluids and antibiotics;
emptied, using a curette very
give ergometrine 0.2mg IM (plus
gently
one or maximum two doses if
• The vacuum has decreased with
bleeding continues), except if
the cannula inside the uterine
omentum or bowel hernia is
cavity
suspected
• Sometimes: excessive bleeding,
• Observe for 24 hours or refer
• Check vital signs and abdominal
atypical pain
• Sometimes: fat, bowel or
signs frequently during the first
omentum observed (refer)
two hours
• If the condition worsens or
bleeding continues under
ergometrine or oxytocin, refer
for surgery
Abdominal pain
• Severe uterine cramps/
contractions
• Acute haematometra? See page
12
• Aspirate liquid and clotted blood
promptly
• Administer an oxytocic and start
an antibiotic
Haemorrhage
• Excessive vaginal blood loss
• Retained products of
• Retention: empty the uterus
conception? Cervical trauma?
• Cervical trauma: five minutes of
Very rare: endometrial angioma
continuous compression. Suture
(can necessitate hysterectomy)
if bleeding does not stop, or
refer
Anaesthesia-related
complications
• See Table 2
• Competent staff must be
• See Table 2
present; ensure narcotic reversal
agents are always available
Vasovagal reaction or syncope
• Fainting, sweating, slow pulse,
slow respiration, nausea,
hypotension, lethargy
• Check blood pressure, pulse,
respiration
• Stop the procedure
• Maintain open airway
• Turn patient to side (to prevent
aspiration of vomitus or saliva)
• Raise the patient’s legs
• If the situation worsens: treat for
shock – see Shock on page 21
Venous air embolism
(very rare)
• Anxiety, pallor, shortness of
breath
• Hypotension and hypoxemia are
found in 10 per cent and 30 per
cent of patients respectively, and
chest pain in 23 per cent
• In general, venous air embolism
is transient and benign
• Give oxygen and administer IV
fluids (rapid flow) without delay
14
Chapter 2 First trimester abortion
Table 5: Mode of action of drugs used for medical abortion, and their side-effects
Drugs
Action
Side-effects
Mifepristone
• An anti-progestin that blocks the endometrial
• Nausea, vomiting, diarrhoea, headache, dizziness, fatigue,
progesterone receptors
tachycardia
• Stops the pregnancy growing, softens the cervix,
increases the uterine contractility, causes bleeding
• A prostaglandin that makes the uterus contract
Misoprostol
• Cramping (more pain than menstrual pain in 25% of patients),
nausea, vomiting, diarrhoea, transient fever, dizziness, headache,
chills, rashes
Methotrexate
• A folic acid antagonist that stops cellular division
• Halts placental development
Gemeprost
• A prostaglandin that makes the uterus contract
• Mild mouth sores, nausea, vomiting, gastro-intestinal
disturbances, hot flushes, fever, chills
• Nausea, vomiting, diarrhoea, transient fever, abdominal pain
Table 6: Regimens for medical abortion and their effectiveness
Regimens
Effectiveness
Use up to
Mifepristone + misoprostol or
>96%
9 weeks from last menstrual period
Misoprostol alone
>83%
12 weeks from last menstrual period
Methotrexate + misoprostol
>90%
9 weeks from last menstrual period
mifepristone + gemeprost
15
Chapter 2 First trimester abortion
• Porphyria.
• Serious pelvic infection (signs: cervical motion tenderness, abnormal pelvic tenderness, adnexal mass, high fever, purulent
cervical/vaginal discharge).
Conditions erroneously cited as contraindications
• Previous C-section(s), previous multiple birth(s), obesity,
fibroids, uterine abnormalities.
• Controversy remains as to whether asthma, heart disease, cardiovascular risk factors, renal and hepatic insufficiency, epilepsy are or
are not contraindications. Referral to a specialist is recommended.
• In breastfeeding women, although data are limited clinicians may advise women not to breastfeed (use pump, and discard
breast milk) up to 72 hours after misoprostol or gemeprost administration.
Counselling
• Discuss the medical abortion methods available and the risks and benefits of each. Explain the side-effects (see Table 5). Explain
sensibly the extremely (but not zero) small risk of major complications/death for all
16
Chapter 2 First trimester abortion
options (there is a degree of risk involved in all medical interventions). The main complications – see Induced abortion:
management of immediate, early and late complications on page 20 – are incomplete abortion, continuing pregnancy,
haemorrhage, infection and adverse psychological sequelae (the latter in a very small
number of women, influenced by pre-existing conditions). Bleeding and cramping indicate that the drugs are working.
• Discuss the symptoms that warrant contacting the on-call provider, which include the following.
– Heavy bleeding (give a criterion that is locally understood, such as soaking two or more big tampons or pads each hour
for two consecutive hours). Explain that with drug-induced abortion, bleeding may be greater than during a heavy
period, and can be accompanied by clots. Ensure a supply of sanitary towels for hygienic management of bleeding. In
very few cases, bleeding will need to be stopped
by a surgical procedure. Spotting may last up to 30 days. Some women may experience a heavy bleeding episode 3–5
weeks after the abortion.
17
Chapter 2 First trimester abortion
– No bleeding at all within 24 hours: after misoprostol,
and if an intrauterine pregnancy was not confirmed
before treatment, ectopic pregnancy must be
considered and managed appropriately.
– Sustained fever (>38°C), or onset of fever in the days
after misoprostol.
– Foul-smelling vaginal discharge.
described as cramping, is most intense during expulsion
and lasts 2–4 hours, after which it usually subsides.
• Bleeding starts in 50 per cent of users before taking
misoprostol, but this drug is typically needed to complete
the expulsion process.
• An embryo is small and is usually not seen before 6–7
weeks from last menstrual period.
– Severe, continuous or increasing abdominal pain.
– “Feeling very sick” including weakness, nausea,
vomiting or diarrhoea, more than 24 hours after taking
Medical history, physical examination, laboratory, ultrasound
See Pre-abortion visit on page 6.
misoprostol.
• Discuss the length of time involved for the abortion to be
Regimens and follow-up
complete and the need for several visits.
• Mifepristone is not teratogenic, but foetal malformations
have been reported after first trimester use of misoprostol
(and of methotrexate). Women must be strongly
advised and motivated to complete the abortion,
medically or using aspiration (or dilatation and
curettage), once the initial medications have been
Disclaimer
See Introduction on page 3. Some descriptions of medical
abortion using mifepristone and misoprostol are partly
based on a National Abortion Federation (NAF) protocol
(March 2006) which reflects the US Food and Drug
Administration (FDA)-approved labelling for mifepristone
and which includes evidence-based alternatives to the
administered. Inform the client that the follow-up visit
FDA-approved regimen. NAF and FDA are not responsible
to confirm complete abortion is essential. Make a formal
for adverse clinical outcomes that might occur in the
appointment for day 14 (earlier visits may be needed if
course of delivery of abortion services. NAF standards
anxiety or complications arise).
and protocols can be found at www.prochoice.org/pubs_
• Vacuum aspiration must be available at any time for a
patient experiencing a delay in the completion of the
research/publications/downloads/professional_education/
cpgs_2007.pdf
expulsion and who prefers aspiration rather than waiting
for the medical abortion to be completed.
• Discuss contraception (see Chapter 5), to be started as
soon as possible after the abortion. The formal
IPPF and World Health Organization recommended
method
• Mifepristone 200mg followed 36–48 hours later by 800µg
appointment on day 14 should be used for checking that
misoprostol (orally, sublingually, buccally or vaginally)14 at
the procedure is complete, and discussing and providing
once or in two doses of 400µg two hours apart,15 up to
contraception.
nine completed weeks after last menstrual period.
• Provide additional explanations:
– Oral administration means swallowing the tablet(s); in
buccal administration the tablets should be retained
between cheek and gum for 30 minutes before
swallowing; sublingual administration means putting
the tablets under the tongue until they disappear.
– Vaginal misoprostol insertion can be done at home:
the client must hand-wash before the insertion. Insert
deeply, and lie still for 30 minutes.
• Pain control: the patient should have oral pain
medications and instructions for use. Pain is typically
US Food and Drug Administration method
• Day 1: Mifepristone 600mg (three 200mg tablets) taken
as a single oral dose.
• Day 2: Rh-negative clients will be administered
Rh-immunoglobulin 50µg (the dose for pregnancies up to
12 weeks), no later than 48 hours after the abortion.
• Day 3: Unless abortion has occurred and is confirmed
by clinical examination (or ultrasonography), administer
misoprostol 400µg (two 200µg tablets) as a single oral
dose.
14 This regimen (with vaginal misoprostol) is recommended by the World Health Organization. The continuing pregnancy rate is around 1 per cent, and
the success rate is more than 96 per cent. See Bibliography, page 32: World Health Organization, 2003.
15 The World Health Organization states that repeated doses of misoprostol increase the side-effects while increased effectiveness is not proven. See
Bibliography, page 32: World Health Organization, 2006.
18
Chapter 2 First trimester abortion
• Day 14: Clinically assess for completion of abortion (or
Always ensure the availability of a back-up clinician to
use ultrasonography or ß-hCG). Vacuum aspiration is
assess possible complications. This is critical with medical
recommended if a viable pregnancy is detected at this
abortion as bleeding is less predictable, and heavy or
time because the pregnancy may continue and there is a
persistent bleeding may occur at home. For treatment,
risk of foetal malformation. Increase of hCG can indicate
vacuum aspiration, uterotonic agents, IV fluid administration
an ongoing pregnancy. If hCG levels have declined by 50
and blood transfusion may be necessary, and can be
per cent in 24 hours, the pregnancy has probably ended.
provided in emergency only in primary health care clinics and
Serum hCG should be below 1,000 IU/L two weeks after
hospitals.
mifepristone administration.
Side-effects of the drugs
Other commonly used evidence-based regimens
See Table 5.
Providers should be guided by accepted medical standards as
well as by local regulations.
Managing complications
• Mifepristone 200mg followed 36–48 hours later by oral
See Induced abortion: management of immediate, early and
misoprostol 400µg, up to seven completed weeks after
late complications on page 20.
last menstrual period (it is less effective after seven
It is essential to have staff trained in re-evacuation of
weeks). After taking the prostaglandin, observe the
the uterus, adequate equipment and a functioning referral
woman for 4–6 hours. Inspect sanitary pads and bedpans
system.
to verify that abortion has taken place. Alternatively,
misoprostol can be taken at home.
• These regimens can be completed with an additional dose
Abortion using misoprostol alone
This regimen is less effective and has more side-effects than
of misoprostol 800µg administered orally or vaginally in
the combination regimens with mifepristone or methotrexate
women with an incomplete abortion on day seven. This is
pre-treatment, but where these drugs are not accessible,
necessary in about 10 per cent of clients. Compared to
medical abortion can be induced if misoprostol is the only
oral misoprostol 400µg, using misoprostol 800µg vaginally
drug available. Effectiveness is lower than for surgical
has fewer gastrointestinal side-effects and increases the
methods (84 per cent compared with 95 per cent). However,
proportion of women with onset of bleeding and likely
the safety level is much higher than resorting to unsafe,
expulsion of pregnancy within four hours of misoprostol
clandestine, illegal abortion.
administration. Although misoprostol is formulated for
oral use, it is more effective if given vaginally or sublingually.
• Mifepristone 200mg, followed 36–48 hours later by
Other regimens have been recommended:
• Repeated doses of misoprostol 800µg, vaginally or
sublingually (oral intake is less efficient), every three
gemeprost 1mg vaginally is also used. Gemeprost is
hours until abortion takes place, but with a maximum of
quite expensive, must be kept frozen and induces more
three doses. With three-hourly intervals the side-effects
side-effects.
• In China, five or six doses of 25mg mifepristone over
are stronger than with 12-hourly intervals (see below).
• Repeated doses of misoprostol 800µg vaginally every 12
three days, followed by a prostaglandin, is used up to
hours until abortion takes place, but with a maximum of
49 days of gestation.
three doses. Sublingual or oral administration at 12-hourly
• Other variants have been tested. Sulprostone and
15-methyl PGF2α are no longer used.
• Home administration of misoprostol has been
found to be safe and effective and is highly acceptable
to patients. If an Rh-negative client is going to take
intervals is not sufficiently effective: 9 per cent of
pregnancies continue if used up to nine weeks’ gestation.
The failure rate is likely to be even higher if administered
up to 12 weeks’ gestation.
• With the two above regimens the complete abortion rate
misoprostol at home, some protocols advise the
is 84 per cent, continued pregnancy rate is around 5 per
administration of Rh-immunoglobulin on day 1. In a
cent, and the incomplete abortion (retained products) rate
gestation of 6–12 weeks, administer 50µg IM; at or
is approximately 11 per cent.
beyond 13 weeks’ gestation, administer 300µg IM.
19
Chapter 2 First trimester abortion
• Other protocols use misoprostol 800µg orally, vaginally or
sublingually, which is repeated if abortion has not started
by day 8, and again at day 11 if abortion has still not
started. Follow-up is on day 21.
• Days 5 to 7: If no bleeding has occurred, administer
misoprostol 800µg orally, sublingually or vaginally.
• Day 9: If no bleeding has occurred, administer a repeat
dose of misoprostol 800µg.
• Day 11: If no bleeding has occurred, administer a third
If Rh-immunoglobulin is provided to Rh-negative women,
dose of misoprostol 800µg. A maximum of three doses
administer 50µg not later than 72 hours after the abortion.
of misoprostol can be administered. If the client does
This is not necessary before six weeks after last menstrual
not abort after the third dose, a surgical procedure is
period.
recommended (preferably aspiration; or a dilatation and
Foetal malformations have been reported after first
curettage if aspiration is not available).
trimester use of misoprostol. Therefore, women must
be strongly advised to complete the abortion, either
If Rh-immunoglobulin is routinely provided to Rh-negative
medically or surgically, once the medication has been
women, administer at the same time as the misoprostol,
administered.
or not later than 48 hours after the abortion. This is not
necessary before six weeks after last menstrual period.
Abortion using methotrexate + misoprostol
available. The combination is more than 90 per cent effective
Induced abortion: management of immediate,
early and late complications
for pregnancies up to nine weeks after last menstrual period.
Staff must be trained and experienced, the equipment
This regimen can be used where mifepristone is not
Once methotrexate is administered, the abortion must be
adequate and the referral system reliable. Staff must be able
completed, because both drugs are teratogenic. Therefore,
to (re-)evacuate a uterus, in cases of incomplete abortion
women must be strongly advised to complete the
or failure of medical abortion. Table 4 describes the early
abortion, either medically or surgically, once the
complications for surgical abortion and their management.
medication has been administered.
See also the World Health Organization publication on
managing complications,16 especially the charts (which can
Contraindications
be printed and displayed on walls) on pages 9, 16, 34, 39
• Severe anaemia.
and 46. Slightly modified versions can be found in Annex 2
• Known coagulopathy.
on pages 35–39 of this publication.
• Acute liver or renal disease.
Complications which can occur after first trimester
• Uncontrolled seizure disorder.
abortion, whatever the technique, are considered next. They
• Acute inflammatory bowel disease.
are failed abortion, acute haematometra, shock, severe
bleeding, infection and intra-abdominal injury.
Administration
• Day 1: Administer methotrexate 75mg IM or 50mg oral.
Continuation of pregnancy (failed abortion) Failed
As with the combined mifepristone + misoprostol regimen,
abortion can occur after surgical and medical attempts; it
give instructions about side-effects, possible
requires a repeat aspiration (preferably) or dilatation and
complications, follow-up and future contraception. After
curettage. There seem to be scarce data, if any, about
administering a prostaglandin (misoprostol, gemeprost or
medical abortion used in cases of failed abortion. Any
other), observe the woman for 4–6 hours. Inspect sanitary
woman presenting with an incomplete abortion may also be
pads and bedpans to verify that abortion has taken place.
experiencing one or more life-threatening
The following next steps can take place at the health
complications: shock, severe vaginal bleeding, infection and
centre or at home, depending on distances and client’s
sepsis (see pages 21–24).
preference.
16 World Health Organization (1994) Clinical Management of Abortion Complications: A Practical Guide,
www.who.int/reproductive-health/publications/clinical_mngt_abortion_complications/text.pdf
20
Chapter 2 First trimester abortion
Acute haematometra (sometimes called post-abortion
syndrome)
Initial treatment
Acute haematometra can occur from a few hours to three
protocols, but start initial treatment immediately.
days after abortion. The uterus is tender and distended by
• Make sure airway is open.
blood, which causes rectal pressure. Cramping is present,
• Check vital signs (pulse, blood pressure, breathing).
together with vagal symptoms (tendency to faint, pallor,
• Secure IV line.
bradycardia, low blood pressure, low respiration, sweating).
• Keep the patient warm.
There is minimal or no vaginal bleeding. The treatment
• Turn body and head to the side (if the woman vomits, she
consists of prompt aspiration of both liquid and clotted
blood, leading to rapid resolution. An oxytocic (misoprostol,
Refer, if necessary, according to local capacities and
is less likely to aspirate).
• Raise legs (or the foot of the bed). However, if this causes
oxytocin or ergometrin) is administered after the repeat
difficulty in breathing, there may be pulmonary oedema
evacuation. Antibiotherapy is added.
or heart failure. In this case, lower the legs and raise the
head to relieve fluid pressure on the lungs.
• Give IV Ringer’s lactate or isotonic solution 1L for 15–20
Shock
Signs
minutes (16–18 gauge needle). It may take 1–3L to
Shock results from blood loss and/or sepsis, pain being an
stabilize a patient in shock. Do not give fluids by
aggravating factor. Oxygen supply to tissues is interrupted.
mouth.
It is a highly unstable condition with a high risk of mortality.
Possible signs are fast (>110/minute) and weak pulse; low
blood pressure; pallor of the skin around the mouth or on
the palms; pale conjunctiva; rapid respiration (>30/minute);
anxiety; confusion; dizziness; loss of consciousness. Treat
immediately, and start IV perfusion at once. Treat the
shock first; then treat the cause.
• If available, give oxygen 6–8L/minute (mask or nasal
cannula).
• Remove any visible products of conception in the cervical
os.
• If haemoglobin is <5g/dL or haematocrit <15%, a blood
transfusion is necessary.
• Strictly monitor the amounts of fluids/blood given (use a
chart).
Table 7: Signs of early and late shock
Signs of early shock (can usually be treated at
the primary health care level)
Signs of late shock (must usually be treated at
referral level)
Pulse
>110/minute
Fast and weak
Blood pressure
Systolic <90mmHg
Very low
Pallor
Inner eyelid, palms, around the mouth
Very pale
Breathing
>30/minute
Very fast and shallow
Awareness
Awake, anxious
Confusion or unconsciousness
Lungs
Clear
Dense
Haemoglobin
≥8g/dL
<8g/dL
Haematocrit
≥26%
<26%
Urine output
≥30mL/h
<30mL/h
Skin
Pale
Cold, clammy, sweating
21
Chapter 2 First trimester abortion
• Monitor urine output. If urine is very dark, if the output is
Severe genital bleeding
decreased or absent, refer. Shock, blood volume
Signs
depression (for example due to haemorrhage or severe
• Moderate to light vaginal bleeding is characterized by
diarrhoea), sepsis, and ureterine damage can cause
clean pad not soaked after five minutes, fresh blood
oliguria or anuria. Rapid correction of the circulating
mixed with mucus, no clots.
volume is necessary, as well as the elimination of the
underlying cause. Infuse quickly with fluids: 50–200mL of
normal saline or lactated Ringer solution over a 10-minute
period.
• If there is infection (fever, chills, pus) give broad spectrum
• Severe bleeding may be caused by trauma (to the vagina,
cervix, uterus), retained products of conception, fibroids
or atony.
• Signs: heavy, bright red blood with or without clots;
blood-soaked pads, towels or clothing; pallor (inner
antibiotics, IV preferably, or IM. No medications by
eyelids, palms, around the mouth); dizziness, syncope,
mouth.
hypotension.
• Laboratory work is helpful but must not cause any delay
in treatment. Request haemoglobin, haematocrit, blood
• There is a risk of shock if bleeding continues: treat shock,
if it develops.
group and rhesus pre-transfusion (type and cross-match),
platelet count and, if available, blood electrolytes, pH,
Initial treatment for severe genital bleeding
urea and/or creatinine.
• Make sure airway is open.
• Check vital signs (pulse, blood pressure, breathing).
Signs of stabilization
• Secure IV line.
• Increasing blood pressure; systolic blood pressure
• Keep the patient warm.
≥100mmHg.
• Heart rate <90/minute.
• Raise the patient’s legs (or the foot of the bed).
• Control bleeding, if possible and as appropriate by use of
• Conscious, reduced confusion/anxiety.
oxytocics, tamponing, uterine massage, emptying the
• Skin colour improves.
uterus surgically (preferably using aspiration), suturing or
• Respiration rate decreases or is <30/minute.
bimanual internal/external compression.
• Urine output increases and is >100mL/4h.
• If available, give oxygen 6–8L/minute (mask or nasal
cannula).
Continuing treatment for shock
• During the following steps prepare concomitantly for
referral; if the situation worsens, refer without delay.
• If patient is not stabilizing after 20–30 minutes:
– continue monitoring, oxygen and IV fluids
– reassess the need for antibiotics
– promptly begin the treatment of underlying cause(s) of
shock
• If patient is not stabilized after two hours, refer
immediately.
• If patient is stabilizing:
– gradually shut off oxygen. If this causes worsening of
some or all the signs in Table 7, turn oxygen back to
6–8L/minute
– decrease IV fluid administration to 1L/6–8h
• Give IV Ringer’s lactate or isotonic solution 1L for 15–20
minutes (16–18 gauge needle). It may take 1–3L to
stabilize a patient who has lost a lot of blood. Do not
give fluids by mouth.
• If haemoglobin is <5g/dL or haematocrit <15%, a blood
transfusion is necessary.
• Strictly monitor the amounts of fluids/blood given (use a
chart).
• Monitor the urine output for decrease or absence. A
darker colour indicates a reduced output. An increase is a
good sign.
• Give IV or IM analgesia for pain.
• If there is infection (fever, chills, pus) give broad spectrum
antibiotics, IV preferably, or IM. No medications by
mouth.
– continue antibiotics if they have been started
• If needed, give tetanus toxoid and tetanus antitoxin.
– treat the underlying cause(s) of shock
• Laboratory work is helpful but must not cause any delay
in treatment. Request haemoglobin, haematocrit, blood
group and rhesus pre-transfusion (type and cross-match),
22
Chapter 2 First trimester abortion
platelet count and, if available, blood electrolytes, pH,
• Abdominal/pelvic pain, distended abdomen, rebound
urea and/or creatinine. A drop in haemoglobin and
tenderness and abnormal tenderness on bimanual pelvic
haematocrit can lag 6–8 hours behind the actual blood
examination.
loss (time required for equilibrium).
• Sub-involution of the uterus.
• Uterine and cervical motion tenderness.
Signs of stabilization
• Increasing blood pressure; systolic blood pressure
≥100mmHg.
• Heart rate <90/minute.
• Skin colour improves.
• An adnexal mass may be present (this could be an
abscess).
• Shoulder pain can be a sign of ectopic pregnancy (this is
due to irritation of the superior part of the peritoneum) or
of intra-abdominal injury.
• Urine output increases and exceeds 100mL/4h.
• Prolonged vaginal bleeding (check for clots or signs of
Continuing treatment for severe vaginal bleeding
• Laboratory tests: raised white blood cell count.
anaemia) or spotting.
• Continue monitoring, oxygen and IV fluids as long as
patient is unstable.
• If patient is stabilizing:
– gradually shut off oxygen. If this causes worsening
(some or all signs in Table 7), turn oxygen back to
6–8L/minute.
– decrease IV fluid administration to 1L/6–8h. Continue
the blood transfusion as programmed and started
– reassess/continue antibiotics, pain control, tetanus
Infection: assessment of the situation
Determine whether there is high or low risk of the patient
developing septic shock (see Table 7).
• Low risk of developing septic shock: mild/moderate
fever (<38.5°C and >36.5°C), stable vital signs
(pulse, blood pressure, breathing), no evidence of
intra-abdominal injury.
• High risk of developing septic shock: temperature
preventive measures if they have been started or need
≥38.5°C or ≤36.5°C, OR second trimester pregnancy OR
to be started.
evidence of intra-abdominal injury (distended abdomen,
decreased bowel sounds, rigid abdomen, rebound
Infection and sepsis
tenderness, nausea, vomiting) OR any evidence of shock
This can be related or unrelated to retained products
(low blood pressure, anxiety, confusion, unconsciousness,
of conception. Infection may be limited to the cervix or
pallor, rapid breathing, weak pulse).
uterus, or there may be generalized sepsis. Immediate
treatment is required in any case. Monitor carefully for
Initial treatment for sepsis
signs of septic shock. Give broad spectrum antibiotics
• Make sure airway is open.
immediately. Take blood for culture. Any woman
• Monitor vital signs (pulse, blood pressure, breathing).
presenting with an infection after abortion may also be
• Give fluids: IV Ringer’s lactate or isotonic solution 1L for
experiencing one or more life-threatening complications:
15–20 minutes (use a 16–18 gauge needle). It may take
shock, severe vaginal bleeding or septicaemia. If abortion is
1–3L to stabilize a patient in shock. Do not give fluids
performed properly under asepsis, infections are rare, but
some, such as septicaemia, can be very dangerous and can
be fatal.
by mouth.
• Strictly monitor the amounts of fluids/blood given (use a
chart).
• If the patient is at high risk of shock, begin IV
Signs and symptoms
antibiotics immediately and treat for shock. Use
• Perform a general physical examination.
broad spectrum antibiotics, effective against Gram
• Chills, high fever, sweats, influenza-like symptoms (can
negative and positive, anaerobic organisms and
be absent, especially in some very dangerous clostridium
infections).
• Mildly low blood pressure.
• Foul-smelling or muco-purulent vaginal discharge.
Chlamydia.
• According to the patient’s vaccination status, give IM
tetanus toxoid and/or tetanus antitoxin.
• Give IV or IM analgesics.
23
Chapter 2 First trimester abortion
• Oxygen is not necessary if the patient is stable and the
Intra-abdominal injury
risk of shock is low. If the patient becomes unstable, and
Perforation during hysterometry (a procedure NOT
if it is available, give oxygen 6–8L/minute (mask or nasal
recommended) and suspected uterine or cervical perforation
cannula).
with incomplete or complete evacuation are considered
• Laboratory work is helpful but must not cause any delay
in treatment. If the patient has lost a lot of blood, assess
earlier (see Table 4).
Perforation of and damage to surrounding organs is
haemoglobin, haematocrit, blood group and rhesus
possible, and carries the risk of infection and sepsis.
pre-transfusion (type and cross-match), complete blood
Differential diagnosis: ruptured ectopic pregnancy, ruptured
count to assess anaemia and infection and the possibility
abscess, ruptured ovarian cyst, acute appendicitis.
of disseminated vascular coagulation, and platelet count
Symptoms that may be present include abdominal
(if there is disseminated vascular coagulation, the platelet
pain, cramping; tense, hard, distended abdomen, rebound
count will be low). If available, ask for blood electrolytes,
tenderness; decreased bowel sounds; nausea, vomiting;
pH, urea and/or creatinine.
shoulder pain; fever; shock; sepsis.
• Monitor urine output for decrease or absence. A darker
colour indicates a reduced output. An increase is a good
Initial treatment for intra-abdominal injury
sign.
• Check vital signs (pulse, blood pressure, breathing).
• If possible take a flat-plate abdominal X-ray to check for
• Check haemoglobin or haematocrit.
air and fluid levels in the bowels, and an upright
abdominal one to check for air under the diaphragm. This
If there are signs of shock (see page 21 and Table 7):
signals uterine or bowel perforation.
• Raise the patient’s legs.
• Treat the underlying cause of infection: re-evacuate
the uterus.
• Check for signs of gas gangrene, tetanus, intra-abdominal
injuries, peritonitis and pelvic abscesses.
• Remove intrauterine device if present.
• Make sure airway is open.
• Do not give anything by mouth.
• If available start oxygen 6–8L/minute.
• Give fluids: IV Ringer’s lactate or isotonic solution 1L for
15–20 minutes (use a 16–18 gauge needle). It may take
1–3L to stabilize a patient who has lost a lot of blood or
Signs of stabilization
• Increasing blood pressure; systolic blood pressure
≥100mmHg.
• Heart rate <90/minute.
is in shock.
• If haemoglobin is <5g/dL or haematocrit <15%, a blood
transfusion is necessary.
• Immediately begin broad spectrum antibiotics IM
• Skin colour improves.
or, preferably, IV. Use antibiotics effective against
• Urine output increases and exceeds 100mL/4h.
Gram negative and positive, anaerobic organisms and
Chlamydia.
Continuing treatment for sepsis
• If necessary, give tetanus toxoid and tetanus antitoxin.
• Continue monitoring vital signs, urine output, fluids/blood
• Give analgesics.
volume administration.
• If oxygen has been given, gradually shut it off. If this
causes deterioration, turn oxygen back to 6–8L/minute.
• If IV fluids were started to administer antibiotics, continue
with the IV treatment.
• Once the fluid volume has been corrected, decrease IV
fluid administration to 1L/6–8h.
• If haemoglobin is <5g/dL or if haematocrit is <15%, a
blood transfusion is necessary.
• Laboratory work is helpful but must not cause any delay
in treatment. Assess haemoglobin, haematocrit, blood
group and rhesus pre-transfusion (type and cross-match),
complete blood count to assess anaemia and infection
and the possibility of disseminated vascular coagulation,
and platelet count (if there is disseminated vascular
coagulation, the platelet count will be low). If available,
ask for blood electrolytes, pH, urea and/or creatinine.
• Monitor urine output for decrease or absence. A darker
colour indicates a reduced output. An increase is a good
sign.
24
Chapter 7 Equipment, instruments, supplies, drugs and medications
• If possible take a flat-plate abdominal X-ray to check for
gas in the peritoneal cavity, and an upright (or, if
impossible, a lateral) abdominal one (the presence of gas
is a sign of uterine or bowel perforation).
• If necessary, remove the retained products of conception,
assessing the complete evacuation of the uterus under
direct visual control (laparoscopy, mini-laparotomy or
ultrasound if available) and assessing damage to the
pelvic organs. Repair the vessels, bowel and other
abdominal organs surgically. If laparotomy or laparoscopy
cannot be performed, stabilize and refer the patient. After
surgery, give oxytocics and observe the vital signs every 15
minutes for two hours; then give ergometrine 0.2–0.5mg
IM and observe overnight. If the patient worsens, refer.
Signs of stabilization and improvement
• Increasing blood pressure; systolic blood pressure
≥100mmHg.
• Heart rate <90/minute.
• Skin colour improves.
• Urine output increases and exceeds 100mL/4h.
Continuing treatment of intra-abdominal injury
• Continue to monitor vital signs, urine output and fluids.
• For surgical repair, refer.
31
Chapter 7 Equipment, instruments, supplies, drugs and medications
Equipment, instruments, supplies,
drugs and medications
Running water, clean toilet facilities, adequate infection prevention techniques and
sensible disposal of conception materials (according to local guidelines and clients’
desires) are essential.
Infection prevention
The following are required:
• sterile gloves (small, medium, large)
• detergent
• soap
• chlorine or glutaraldehyde
• large containers for the above
• high level disinfection or sterilization agent
• water-based antiseptic solution for cleaning vulva and
vagina
• small container for antiseptic solution
• cotton swabs or gauze sponges
Aspiration using manual vacuum aspiration
syringe or electric vacuum pump
The following are required:
• syringes (5, 10, 20mL)
• needles:
– 22 gauge spinal for cervical block
– 21 gauge for drug administration
• speculum
• tenaculum
• sponge (ring) forceps
• dilators (Hegar: sizes 4–14mm)
• vacuum aspirator (manual vacuum aspiration syringe or
electric pump + tube)
• flexible cannulas (sizes 6–12 or 14) plus adaptors, if
needed
• silicone for lubricating syringes, if needed
• medications:
– analgesics (for example acetaminophen, ibuprofen,
pethidine)
31
Chapter 7 Equipment, instruments, supplies, drugs and medications
– lidocaine or similar: 0.5, 1 or 2 per cent without
epinephrine
– oxytocin 10 International Units
– ergometrine 0.2mg
• strainer (or gauze)
• clear glass dish for inspecting products of conception
• all contraceptives (including emergency contraception)
used locally
Dilatation and curettage
The following are required:
• sterile gloves (small, medium, large)
• infection prevention: see left
• speculums of various sizes (Collin is preferred rather than
Sims, Cusco, Graves or valve(s))
• sponge (ring) holding forceps
• tenaculum forceps
• volsellum
• set of Hegar dilators
• set of three curettes of different diameters
• gauze, compresses
• antibiotics
Medical (drug-induced) abortion
The following are required:
• mifepristone or methotrexate and misoprostol, or
misoprostol alone
• antibiotics: metronidazole, doxycycline
• analgesics: paracetamol (= acetaminophen), ibuprofen,
codeine
• uterotonics: injectable ergometrine; injectable oxytocin
• iron tablets
31
Chapter 7 Equipment, instruments, supplies, drugs and medications
Emergency room drugs, supplies and posters
The following are required:
• sphygmomanometer, stethoscope, thermometer, torch
• gloves, syringes, needles
• IV infusion set, IV fluids (sodium lactate, glucose, saline)
• emergency bag with resuscitation equipment and oxygen
• vaginal speculums
• spatulae for vaginal/cervical mucus/pus
• refrigerator
• autoclave
• antibiotics, analgesics, diazepam, epinephrine (adrenaline)
1:1,000 1mg/mL
• emergency protocols poster to display on the wall (so all
staff know what to do)
31
Annex 2 Clinical management of abortion complications
Pre-abortion ultrasound and
laboratory testing
Ultrasound
This is not needed routinely. Use according to local protocols. Ultrasound should not be a prerequisite for abortion.
Ultrasound can be useful (but is not 100 per cent sensitive or specific) to confirm pregnancy duration, detect an ectopic
pregnancy, or show a molar pregnancy (‘snow storm’ appearance). If ultrasound is indicated (not routinely), it is better to use
a vaginal probe than an abdominal one. Look for the gestational sac, the yolk sac, the embryonic pole and the presence of
cardiac activity. If an embryonic pole is visible, use its measurement to estimate the gestation duration as it is more accurate
than the measurement of the gestational sac. The absence of an intrauterine sac could indicate early intrauterine pregnancy,
ectopic pregnancy or an abnormal intrauterine pregnancy. With no intrauterine sac seen (using a vaginal probe) and a serum
ß-hCG >2,000mIU/mL, an ectopic pregnancy must be considered. The same applies if ß-hCG is >3,600 and an abdominal
probe is used. In such cases further evaluation and/or treatment is warranted.
Laboratory testing
Laboratory testing should not be a prerequisite for abortion. There are no essential tests.
• A pregnancy test is not required routinely; only if the pregnancy is uncertain.
• Haemoglobin/haematocrit if anaemia is suspected.
• ABO-Rh where available is useful if transfusion can be accessed. Certainly needed if Rh-immunoglobulin is available and
provided (this is best practice) to Rh-negative women (within 48–72 hours of abortion; unnecessary before six weeks’
gestation).
• HIV test, other tests for sexually transmitted infections or tests related to specific individual health problems can be
proposed or may be necessary.
• Cervical cultures should be performed according to local protocols, or if signs of vaginal/cervical infection are present.
• Screening for cervical cancer can be done if facilities exist.
35
Annex 2 Clinical management of abortion complications
Annex 2
Clinical management of abortion
complications
Chart 1: Initial assessment
Presentation
Initial step
If there are signs of shock
After an abortion, or in a woman of
reproductive age possibly having been
pregnant, in presence of:
• excessive vaginal bleeding
• cramping or lower abdominal pain
• fever
• signs of shock
Assess for shock
• Rapid, weak pulse
• Low blood pressure
• Pale and sweaty
• Rapid breathing
• Anxious, confused or unconscious
Immediate action is required
(see page 21 and Table 7)
Complete clinical assessment
Review history:
Length of amenorrhoea/last menstrual period, duration and amount of bleeding, duration and severity of
cramping, abdominal pain, shoulder pain, drug allergies
Physical examination:
Vital signs, heart, lungs, abdomen, extremities
Indication of systemic problem (shock, sepsis etc)
Pelvic examination:
Uterine size, stage of abortion, uterus position
Other:
Remove any visible products of conception from the cervical os
Determine rhesus status if possible
Shock
Early
• Pulse >110/minute
• BP <90mmHg systolic
• Pale and sweaty skin
• Breathing >30/minute
• Awake
• Anxious
• Lungs clear
• Haematocrit ≥26%
• Urine output ≥30mL/h
See Chart 2
Late
• Weak pulse, very rapid
• BP very low
• Pale and cold skin
• Breathing rapid
• Unconscious
• Confused
• Pulmonary oedema
• Haematocrit <26%
• Urine output <30mL/h
Intra-abdominal
injury
• Abdominal pain,
cramping
• Distended abdomen
• Decreased bowel
sounds
• Tense, hard abdomen,
rebound tenderness
• Nausea, vomiting
• Shoulder pain
• Fever
See Chart 4
Infection and sepsis
• Chills, fever, sweats
• Foul-smelling vaginal
discharge
• History of interference
with the pregnancy
• Abdominal pain
• Intrauterine device in
place?
• Prolonged bleeding
• Influenza-like symptoms
See Chart 5
Severe vaginal
bleeding
• Heavy, bright red vaginal
bleeding with or without
clots, blood soaked pads,
towels, clothing
• Pallor
See Chart 3
35
Annex 2 Clinical management of abortion complications
Chart 2: Shock
Initial treatment
Shock
Early
• Pulse >110/minute
• BP <90mmHg systolic
• Pale and sweaty skin
• Breathing >30/minute
• Awake
• Anxious
• Lungs clear
• Haematocrit ≥26%
• Urine output ≥30mL/h
Late
• Weak pulse, very rapid
• BP very low
• Pale and cold skin
• Breathing rapid
• Unconscious
• Confused
• Pulmonary oedema
• Haematocrit <26%
• Urine output <30mL/h
• Make sure airway is open
• Check vital signs (pulse, blood pressure, breathing)
• Secure IV line: Ringer’s lactate or isotonic solution 1L for 15–20 minutes (16–18 gauge
needle). It may take 1–3L to stabilize a patient in shock
• Turn body and head to the side, raise legs (however, if this causes difficulty in breathing,
lower legs and raise head)
• No fluids by mouth
• Give oxygen 6–8L/minute (if available)
• Remove any visible products of conception from the cervical os
• Blood transfusion: if haemoglobin <5g/dL or haematocrit <15%
• Monitor amount of fluids/blood given – use a chart
• Monitor urine output – colour and quantity
• IV antibiotics – in case of infection
• No medications by mouth
• Laboratory work is helpful but must not cause any delay in treatment. Request haemoglobin,
haematocrit, blood group and rhesus pre-transfusion (type and cross-match), platelet count
and, if available, blood electrolytes, pH, urea and/or creatinine.
Assess response to fluids after 20–30 minutes
Signs of stabilization
• Increasing systolic BP, ≥100mmHg
• Heart rate <90/minute
• Conscious, reduced confusion/anxiety
• Skin colour improves
• Respiration rate ≤30/minute
• Urine output >100mL/4h
If stable
If not stable
• Gradually shut off oxygen. If this
causes worsening, turn oxygen
back on 6–8L/minute
• Clinical assessment
• Uterine evacuation
• Continue monitoring oxygen and IV fluids
• Reassess the need for antibiotics
• Treat underlying cause(s) of shock
If stable
Assess after two hours
• Adjust IV and oxygen
• Complete clinical assessment
• Continue treating underlying
cause(s) of shock
• Uterine evacuation
Signs of stabilization
• Increasing systolic BP, ≥100mmHg
• Heart rate <90/minute
• Conscious, reduced confusion/anxiety
• Skin colour improves
• Respiration rate ≤30/minute
• Urine output >100mL/4h
If not stable
Refer to secondary or tertiary hospital
Ref: WHO/FHE/MSM/94.1
35
Chart 3: Severe vaginal bleeding
Presentation
Initial treatment
• Heavy, bright red blood from
vagina, with or without clots
• Blood soaked pads, towels or
clothing
• Pallor
• Dizziness
• Syncope
• Hypotension
• Make sure airway is open
• Check vital signs (pulse, blood pressure, breathing)
• Secure IV line
• Keep the patient warm
• Raise the patient's legs
• Control bleeding (if possible by use of oxytocics, tamponing, uterine
massage, emptying the uterus by aspiration, suturing or bimanual
internal/external compression)
• Oxygen 6-8Uminute (if available)
• IV Ringer's lactate or isotonic solution 1 L for 15-20 minutes (16-18
gauge needle). It may take 1-3L to stabilize a patient who has lost a lot
of blood
• Do not give fluids by mouth
• Blood transfusion if haemoglobin <5g/dolr haematocrit <15%
• Monitor amount of fluids/blood given -use a chart
• Monitor urine output: dark colour indicates reduced output. Increase is
a good sign
• If infection is present,give antibiotics IV or IM
• No medications by mouth
• If needed, give tetanus toxoid and tetanus antitoxin
• Laboratory work is helpful but must not cause any delay in treatment.
Request haemoglobin,haematocrit, blood group and rhesus
pre-transfusion (type and cross-match), platelet count and, if available,
blood electrolytes, pH, urea and/or creatinine. A drop in haemoglobin
and haematocrit can lag 6-8 hours behind the actual blood loss.
Signs of stabilization
•
•
•
•
BP, systolic ;o-1OOmmHg
Heart rate <90/minute
Skin colour improves
Urine output >1OOmU4h
Suspicion of intraabdominal injury
• Rigid abdomen, acute
abdominal pain
• Immediate assessment
is required
• Emergency laparotomy
may be indicated
See Chart 4
Visible cervical or
vaginal laceration
Incomplete abortion
• Cervix open
• Uterine size is smaller or=
dates of la st menstrual period
-+
• Give IV or IM analgesics
for pain
• Repair laceration
• Continue monitoring oxygen,
IV fluids
• Determine uterine size at
presentation
If no IV facilities:
•
•
•
•
Pain control
Uterine evacuation
Uterine massage
Prepare for referral
Evidence of uterine
perforation
---+- •
Instrument extends beyond
uterus
• Fat or bowel in specimen
• Management depends on
whether or not evacuation is
complete. If not, evacuate
• Continue IV fluids
Evacuate uterus
<;14 weeks
>1 4 weeks
Manual vacuum aspiration or dilatation and
curettage
Misoprostol or dilatation and curettage
Ref:WH0/FHE/MSM/94.1
Chart 4: Intra-abdominal injury
Suspected or confirmed uterine or cervical
perforation during electric vacuum aspiration/manual
vacuum aspiration/dilatation and curettage, or
hysterometry (a procedure NOT recommended)
Suspected or confirmed abdominal injury
detected post-abortion
Signs of shock
Early
• Pulse >110/minute
• BP <90mmHg systolic
• Pale and sweaty skin
• Breathing >30/minute
• Awake
• Anxious
• Lungs clear
• Haematocrit ≥26%
• Urine output ≥30mL/h
See Chart 2
Late
• Weak pulse, very rapid
• BP very low
• Pale and cold skin
• Breathing rapid
• Unconscious
• Confused
• Pulmonary oedema
• Haematocrit <26%
• Urine output <30mL/h
Management of shock
NO
YES
YES
See Chart 2
NO
Is uterus empty?
Verify by gently using a curette
YES
• Observe for 24 hours
• Ergometrine 0.2mg IM
Transfer to tertiary health care/
hospital preferably to obstetrics
and gynaecology unit
NO
•
•
•
•
Haemorrhage
Fever
Severe pain
Signs of shock
• IV Ringer’s lactate or isotonic
solution 1L for 15–20 minutes
(16–18 gauge needle)
• Antibiotics IV/IM
YES
NO
Client can be discharged
Chart 5: Infection and sepsis
Presentation
Initial assessment of risk for septic shock
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Chills, high fever,sweats,influenza-like symptoms
Mildly low blood pressure
Foul-smelling or muco-purulent vaginal discharge
Abdominal/pelvic pain, distended abdomen
Rebound tenderness
Sub-involution of the uterus
Uterine and cervical motion tenderness
Shoulder pain
Prolonged vaginal bleeding
Length of gestation
Check vital signs
Check for signs of pelvic infection
Foreign materials in vagina
Pus in cervix or vagina
Evidence of local pelvic infection
- adnexal tenderness
- uterine tenderness
- cervical motion tenderness
- lower abdominal tenderness
- foul odour to any blood or secretions,urine,faeces
I
Low risk of septic shock
High risk of septic shock
• Mild/moderate fever <38.5°C and >36.5°C
• Vital signs stable
• No evidence of intra-abdominal injury
• Fever >38.5°C or <36.5°C
• Evidence of intra-abdominal injury (distended abdomen,
rebound tenderness)
• Nausea and vomiting
• Low blood pressure
• Anxiety, confusion, pallor, rapid breathing,weak pulse
• Unconscious
Initial treatment
Initial treatment
• Make sure airway is open
• Monitor vital signs (pulse,blood
pressure,breathing)
• IV fluids: Ringer's lactate or isotonic
solution 1 L for 15-20 minutes
• Do not give fluids by mouth
• Antibiotics (IV preferred)
• Tetanus toxoid and tetanus antitoxin
• Analgesics IM
• Make sure airway is open
• Check vital signs (pulse,blood pressure,breathing)
• Secure IV line: Ringer's lactate or isotonic solution 1L for 15-20 minutes (16-18 gauge needle).
It may take 1-3L to stabilize a patient in shock
• Turn body and head to the side,raise legs (however,if this causes difficulty in breathing, lower
legs and raise head)
• No fluids by mouth
• Give oxygen 6-8Uminute (if available)
• Remove any visible products of conception from the cervical os
• Blood transfusion: if haemoglobin <5g/dL or haematocrit <15%
• Monitor amount of fluids/blood given -use a chart
• Monitor urine output- colour and quantity
• IV antibiotics- in case of infection
• No medications by mouth
• Abdominal X-ray- to detect uterine or bowel perforation
• Laboratory work is helpful but must not cause any delay in treatment. If the patient has lost a
lot of blood, assess haemoglobin, haematocrit, blood group and rhesus pre-transfusion (type
and cross-match), complete blood count to assess anaemia and infection and the possibility
of disseminated vascular coagulation,and platelet count (if there is disseminated vascular
coagulation,the platelet count will be low). If available,ask for blood electrolytes, pH,urea
and/or creatinine
I
J
If patient is
stable
• Continue
antibiotics
and IV
• Uterine
evacuation
• Observe for
48 hour s
If signs of
disseminated
vascular coagulation
are present
• Blood does not clot
• Bleeding from
veni-puncture sites etc
• Uterine evacuation
• Blood transfusion
J
J
J
Signs of gas gangrene
or tetanus
Signs of
intra-abdominal injury
If signs of shock
develop
• Gas gangrene: X-ray shows
gas in pelvis
• Tetanus: painful muscle
contractions, generalized
spasms,convulsions
• Refer to tertiary care
centre after initial
stabilizing efforts +
• X-ray shows air in the
abdomen,abdomen rigid,
rebound tenderness
• Dropping blood
pressure
• Fast,weak pulse
• Continue oxygen, antibiotics
and IV fluids
• Emergency Iaparotomy,
or refer immediately to
tertiary care centre
• Rapid breathing
• Pallor
Immediate attention
is required
• Refer to tertiary care
antibiotics and sedation if
See Chart 4
CHILDBIRTH / OBSTETRICAL EMERGENCIES
ABNORMAL BIRTH EMERGENCIES
UNIVERSAL PATIENT CARE PROTOCOL
B
EMT – B
B
I
P
M
EMT – I
EMT – P
MED CONTROL
I
P
M
CORD
AROUND
NECK
PROLAPSED
CORD
BREECH
BIRTH
SHOULDER
DYSTOCIA
Loosen cord or
clamp and cut if
too tight
Transport
mother with
hips elevated
and knees to
chest
Transport
unless delivery
is imminent
Transport
mother with
hips elevated
and knees to
chest
Continue
Delivery
Insert fingers to
relieve pressure
on cord
Do not
encourage
mother to push
Insert fingers to
relieve pressure
on cord
Support but do
not pull
presenting parts
Place pressure
on symphisis
pubis
If delivery is in process and the head is
caught inside vagina, create air passage
by supporting body of infant and placing
2 fingers along sides of nose, and push
away from face to facilitate an airway
passage
If unable to deliver, transport mother
with hips elevated and knees to chest
CONTACT MEDICAL CONTROL
TRANSPORT
CHILDBIRTH / OBSTETRICAL EMERGENCIES
ABNORMAL BIRTH EMERGENCIES
CONTACT MEDICAL CONTROL IMMEDIATEL WHEN ANY ABNORMAL BIRTH
PRESENTATION IS DISCOVERED
HISTORY
•
•
•
•
•
•
Past medical history
Hypertension meds
Prenatal care
Prior pregnancies / births
Gravida / Para
Ultrasound findings in
prenatal care
SIGNS AND SYMPTOMS
•
•
•
•
•
Frank Breech (buttocks
presents first)
Footling Breech (one foot or
both feet presenting)
Transverse Lie (fetus is on
his/her side with possible
arm or leg presenting)
Face First Presentation
Prolapsed Cord (umbilical
cord presents first)
DIFFERENTIAL DIAGNOSIS
•
•
Miscarriage
Stillbirth
KEY POINTS
General Information
•
DO NOT pull on any presenting body parts.
•
These patients will most likely require a c-section, so immediate transport is needed.
•
Prolonged, non-progressive labor distresses the fetus and mother. Be sure to reassess mother’s vital signs
continuously.
Cord Around Baby's Neck
•
As baby's head passes out of the vaginal opening, feel for the cord. Initially try to slip cord over baby’s head; if too
tight, clamp cord in two places and cut between the clamps.
Breech Delivery
•
Footling Breech (one or both feet delivered first)
•
Frank Breech (buttocks first presentation)
• When the feet or buttocks first become visible, there is normally time to transport patient to nearest facility.
• If upper thighs or the buttock have come out of the vagina, delivery is imminent.
• If the child's body has delivered and the head appears caught in the vagina, the EMT must support the child's
body and insert two fingers into the vagina along the child's neck until the chin is located. At this point, the
two fingers should be placed between the chin and the vaginal canal and then advanced past the mouth and
nose.
• After achieving this position a passage for air must be created by pushing the vaginal canal away from the
child's face. This air passage must be maintained until the child is completely delivered.
Prolapsed Cord
•
When the umbilical cord passes through the vagina and is exposed, the EMT should check cord for a pulse.
•
The patient should be transported with hips elevated or in the knee chest position and a moist dressing placed
around the cord.
•
If umbilical cord is seen or felt in the vagina, insert two fingers to elevate presenting part away from cord, distribute
pressure evenly when the occiput presents.
•
DO NOT attempt to push the cord back. High flow oxygen and transport IMMEDIATELY.
Shoulder Dystocia
•
Following delivery of the head, the shoulder(s) become “stuck” behind the symphysis pubis or sacrum of the mother.
•
Occurs in approximately 1% of births.
Excessive Bleeding Pre-Delivery
•
If bleeding is excessive during this time and delivery is imminent, in addition to normal delivery procedures, the EMT
should use the Hypovolemic Shock Protocol.
•
If delivery is not imminent, patient should be transported on her left side and Shock Protocol followed.
Excessive Bleeding Post-Delivery
•
If bleeding appears to be excessive, start IV of saline.
•
If placenta has been delivered, massage uterus and put baby to mother's breast.
•
Follow Hypovolemic Shock Protocol.
CHILDBIRTH / OBSTETRICAL EMERGENCIES
OBSTETRICAL EMERGENCIES
B
EMT – B
B
I
P
M
EMT – I
EMT – P
MED CONTROL
I
P
M
UNIVERSAL PATIENT CARE PROTOCOL
MONITOR/IV PROTOCOL
Vaginal Bleeding / Abdominal Pain?
No
Yes
Hypertension?
Known Pregnancy / Missed Period?
Yes
No
Yes
Mild Pre-eclampsia – (BP >140/90, Peripheral
Edema)
1st Trimester –
Miscarriage, Ectopic
Pregnancy
Severe Pre-eclampsia – (BP >140/90, Edema,
Headache, Visual Disturbances)
Eclampsia – Seizures – other signs absent
No
2nd & 3rd Trimester –
Placeta Previa
Abruptio Placenta
Yes
Magnesium sulfate 1.0 to 4.0 grams slow IV
push over 2-3 minutes
Versed 5 mg IV/IM/Nasal
Call Medical Control for Valium Order
Rapid Transport
NORMAL SALINE
IV, 500 cc BOLUS
Pad bleeding, save
and bring with
patient
Childbirth Imminent
Delivery Protocol
Rapid Transport
CONTACT MEDICAL CONTROL
No
See Abdominal
Pain Protocol
Transport
CHILDBIRTH / OBSTETRICAL EMERGENCIES
OBSTETRICAL EMERGENCIES
SIGNS AND SYMPTOMS
DIFFERENTIAL DIAGNOSIS
HISTORY
•
•
•
•
•
Past medical history
Hypertension meds
Prenatal care
Prior pregnancies / births
Gravida / Para
•
•
•
•
•
•
•
Vaginal bleeding
Abdominal pain
Seizures
Hypertension
Severe headache
Visual changes
Edema of hands and face
KEY POINTS
•
•
•
•
Pre-eclampsia / Eclampsia
Placenta previa
Placenta abruptio
Spontaneous abortion
General Information
• Exam: Mental Status, Abdomen,
Heart, Lungs, Neuro.
• May place patient in a left lateral position to minimize risk of supine hypotensive syndrome if in late 2nd or 3rd
trimester.
• Ask patient to quantify bleeding - number of pads used per hour.
• Any pregnant patient involved in a MVC should be seen immediately by a physician for evaluation and fetal
monitoring.
• DO NOT apply packing to the vagina.
• Be alert for fluid overload when administering fluids.
• Consider starting a second IV if the patient is experiencing excessive vaginal bleeding or hypotension.
• Transport to an appropriate OB facility if the
patient is pregnant. Abortion/Miscarriage
• The patient may be complaining of cramping, nausea, and vomiting.
• Be sure to gather any expelled tissue and transport it to the receiving facility.
• Signs of infection may not be present if the abortion/miscarriage was recent.
• An abortion is any pregnancy that fails to survive over 20 weeks. When it occurs
naturally, it is commonly called a” miscarriage.” Abruptio Placenta
• Usually occurs after 20 weeks.
• Dark red vaginal bleeding.
• May only experience internal bleeding.
• May complain of a “tearing” abdominal pain
Ectopic Pregnancy
• The patient may have missed a menstrual period or had a positive pregnancy test.
• Acute unilateral lower abdominal pain that may radiate to the shoulder.
• Any female of childbearing age complaining of abdominal pain is considered to have an
ectopic pregnancy until proven otherwise. Pelvic Inflammatory Disease
• Be tactful when questioning the patient to prevent embarrassment.
• Diffuse back pain.
• Possibly lower abdominal pain.
• Pain during intercourse.
• Nausea, vomiting, or fever.
• Vaginal discharge.
• May walk with an altered gait due to
abdominal pain. Placenta Previa
• Usually occurs during the last trimester.
• Painless.
• Bright red vaginal
bleeding. Post Partum
Hemorrhage
• Post partum blood loss greater than 300-500 ml.
• Bright red vaginal bleeding.
• Be alert for shock and
hypotension. Uterine Inversion
• The uterine tissue presents from the vaginal canal.
• Be alert for vaginal bleeding
and shock. PreEclampsia/Eclampsia
• Severe headache, vision changes, or RUQ pain may indicate pre - eclampsia.
• In the setting of pregnancy, hypertension is defined as a BP greater than 140 systolic and greater than 90
diastolic, or a relative increase of 30 systolic and 20 diastolic from the patient's normal (pre-pregnancy)
blood pressure.
Uterine Rupture
• Often caused by prolonged, obstructed, or non-progressive labor.
• Severe
abdominal pain. Vaginal
Bleeding
• If the patient is experiencing vaginal bleeding, DO NOT pack the vagina, pad on outside only.
CHILDBIRTH / OBSTETRICAL EMERGENCIES
UNCOMPLICATED DELIVERY
UNIVERSAL PATIENT CARE PROTOCOL
Observe Head Crowning
Contact Medical Control to Notify of Delivery
Prepare Patient for Delivery
Set-Up Equipment
IV Protocol if Time
Run Normal Saline at 150 ml/hour
Delivery of Head
Firm, gentle pressure with flat of hand to slow expulsion.
Allow head to rotate normally, check for cord around neck, and
wipe face free of debris.
Suction mouth and nose with bulb syringe.
Delivery of Body
Place one palm over each ear with next contraction gently move
downward until upper shoulder appears.
Then lift up gently to ease out lower shoulder Support the head
and neck with one hand and buttocks with other.
REMEMBER THE NEWBORN IS SLIPPERY
Newborn and Cord
Keep newborn at level of vaginal opening. Keep warm and dry.
After 10 seconds, clamp cord in two places with sterile equipment at
least 6-8” from newborn. Cut between clamps.
Allow placenta to deliver itself but do not delay transport waiting.
DO NOT PULL ON CORD TO DELIVER PLACENTA. Take
placenta to hospital with patient.
CONTACT MEDICAL
CONTROL
TRANSPORT
B
EMT – B
B
I
P
M
EMT – I
EMT – P
MED CONTROL
I
P
M
Facilitator’s Guide
CHILDBIRTH / OBSTETRICAL EMERGENCIES
UNCOMPLICATED DELIVERY
CONTACT MEDICAL CONTROL IMMEDIATELY WHEN DELIVERY IS IMMINENT
SIGNS AND SYMPTOMS
DIFFERENTIAL DIAGNOSIS
HISTORY
•
•
•
•
•
•
•
Due date
Time contractions started /
how often
Rupture of membranes
Time / amount of any
vaginal bleeding
Sensation of fetal activity
Past medical and delivery
history
Medications
•
•
•
•
•
•
Spasmotic pain
Vaginal discharge or
bleeding
Crowning or urge to push
Meconium
Left lateral position
Inspect perineum
(No digital vaginal exam)
•
•
•
Abnormal presentation
• Buttock
• Foot
• Hand
• Prolapsed cord
Placenta previa
Abruptio placenta
KEY
POIN
TS
•
•
•
•
•
•
•
•
•
•
•
Exam (of Mother): Mental Status, Heart, Lungs, Abdomen, Neuro.
Document all times (delivery, contraction frequency, and length).
If maternal seizures occur, refer to the Obstetrical Emergencies Protocol.
After delivery, massaging the uterus (lower abdomen) will promote uterine contraction and help
to control post-partum bleeding.
Some perineal bleeding is normal with any childbirth. Large quantities of blood or free
bleeding are abnormal.
Prepare to deliver on scene (protecting the patient’s privacy). If delivery becomes imminent
while enroute, stop the squad and prepare for delivery.
Newborns are very slippery, so be careful not to drop the baby.
There is no need to wait on scene to deliver the placenta.
If possible, transport between deliveries if the mother is expecting twins.
Allow the placenta to deliver, but DO NOT delay transport while waiting.
DO NOT PULL ON THE UMBILICAL CORD WHILE PLACENTA IS DELIVERING.
]
Facilitator’s Guide
Activity: Individual work to review signs and symptoms associated with the most common
causes of PPH.
Mini
Mini case
case studies
studies
AT3-15
AT3-15
• Find learning activity 2: Assessing excessive vaginal
• Find learning activity 2: Assessing excessive vaginal
bleeding after childbirth, found in the classroom
bleeding after childbirth, found in the classroom
learning activities for Additional Topic 3: Managing
learning activities for Additional Topic 3: Managing
complications during the third stage of labor in the
complicationsNotebook.
during the third stage of labor in the
Participant’s
Participant’s Notebook.
• Read through each mini case study and decide on
• Read through each mini case study and decide on
the most probable cause of each woman’s
the most probable cause of each woman’s
postpartum hemorrhage.
postpartum hemorrhage.
• You may refer to Table 8 in Additional Topic 3:
• You may refer to Table 8 in Additional Topic 3:
Managing complications during the third stage of
Managing
during the third stage of
labor
in thecomplications
Reference Manual.
labor in the Reference Manual.
Notes to the facilitator:
•
Ask participants to turn to
classroom learning activity 2:
Assessing excessive vaginal
bleeding after childbirth for
Additional Topic 3: Managing
complications during the third
stage of labor in the
Participant’s Notebook.
•
They should also refer to Table 8
in Additional Topic 3 in the
Reference Manual.
•
Give participants 5 minutes to
read the mini case studies and
write in their answers.
Notes to the facilitator:
•
After they have had time to review the mini case studies, put the correct answers up
(remember that the answer provides the MOST probable diagnosis).
•
Ask participants if their answers agree with yours. Facilitate a discussion if there are
questions about the probable diagnosis.
•
Answer any questions.
Mini
Mini case
case studies
studies
Answers
Answers
••
Ms.
Ms. A:
A: Retained
Retained placenta
placenta
••
Ms.
Ms. B:
B: Genital
Genital lacerations
lacerations
••
Ms.
Ms. C:
C: Cervical
Cervical tear
tear
••
Ms.
Ms. D:
D: Retained
Retained placental
placental fragments
fragments ++
uterine
atony
uterine atony
••
Ms.
Ms. E:
E: Inverted
Inverted uterus
uterus
••
Ms.
Ms. F:
F: Uterine
Uterine atony
atony
AT3-16
AT3-16
Facilitator’s Guide
Flipchart / Overhead / PowerPoint slides 17 and 18
Time: 15 min.
Activity: Illustrated lecture to review management of uterine atony.
Objective: Describe the immediate medical management of the woman whose uterus does
not contract adequately.
Notes to the facilitator:
•
Ask participants to turn to the
section What if the uterus
does not adequately contract?
in Additional Topic 3 of the
Reference Manual and refer
to management of uterine
atony.
•
Present the steps to follow
when a woman presents with
uterine atony (if possible,
perform a simulation of the
steps as presented in the
Reference Manual).
•
Remind participants that
uterine atony is the leading
cause of PPH.
Uterine
Uterine Atony:
Atony: Management
Management
•• Continue
Continue to
to massage
massage the
the
uterus
uterus
•• Use
Use uterotonic
uterotonic drugs
drugs
• Perform bimanual
• Perform bimanual
compression
compression
• Perform aortic
• Perform aortic
compression
compression
• Surgical management
• Surgical management
AT3-17
AT3-17
At
At all
all times:
times:
••Anticipate
Anticipate need
need for
for
blood
blood and
and transfuse
transfuse
as necessary.
as necessary.
• Consider other
• Consider other
causes / contributing
causes / contributing
factors – genital
factors – genital
lacerations, retained
lacerations, retained
placenta, clotting
placenta, clotting
disorder.
disorder.
Packing the uterus is ineffective and
Packingwastes
the uterus
is ineffective
and
precious
time.
wastes precious time.
•
Emphasize that uterine atony
may coexist with genital lacerations, retained placental fragments, retained placenta,
and clotting disorders.
•
Emphasize that packing the uterus is ineffective and wastes precious time.
Notes to the facilitator:
•
Review uterotonic drugs to administer to manage uterine atony (see Table 9 in the
Reference Manual).
Uterotonics for treatment of PPH
Oxytocin
Dose and
route
IV: Infuse 20 units
in 1 L IV fluids at
60 drops per
minute
Ergometrine
IM: give 0.2 mg
AT3-18
Misoprostol
1,000 mcg rectally
IM: 10 units
Continuing
dose
IV: Infuse 20 units
in 1 L IV fluids at
40 drops per
minute
Maximum
dose
Not more than 3 L
of IV fluids
containing
oxytocin
Repeat 0.2 mg IM
after 15 minutes
If required, give
0.2 mg IM every 4
hours
5 doses (total 1.0
mg)
Not known
Oral dose should
not exceed 600
mcg because of
risk of fever
Facilitator’s Guide
Activity: Demonstration of a bedside clotting test.
Notes to the facilitator:
•
Demonstrate how to perform a bedside clotting test to rule out a blood clotting
disorder.
•
Remind participants that they should also consider the possibility that the woman
has a clotting disorder any time a woman has PPH.
Bedside
Bedside clotting
clotting test
test
AT3-19
AT3-19
•• Take
Take 22 mL
mL of
of venous
venous blood
blood into
into aa small,
small, dry,
dry,
clean
clean plain
plain glass
glass test
test tube
tube (approximately
(approximately 10
10
mm
mm xx 75
75 mm).
mm).
•• Hold
Hold the
the tube
tube in
in your
your closed
closed fist
fist to
to keep
keep itit
warm
warm (+37°
(+37°C).
C).
•• After
After 44 minutes,
minutes, tip
tip the
the tube
tube slowly
slowly to
to see
see ifif aa
clot
clot is
is forming.
forming. Then
Then tip
tip itit again
again every
every minute
minute
until
until the
the blood
blood clots
clots and
and the
the tube
tube can
can be
be
turned
turned upside
upside down.
down.
•• Failure
Failure of
of aa clot
clot to
to form
form after
after 77 minutes
minutes or
or aa
soft
soft clot
clot that
that breaks
breaks down
down easily
easily suggests
suggests aa
blood-clotting
blood-clotting disorder.
disorder.
Flipchart / Overhead / PowerPoint slides 20 and 21
Time: 20 min.
Activity: Demonstration and return demonstration of bimanual compression of the uterus.
Notes to the facilitator:
•
Ask participants to stand around the model to observe the demonstration of internal
bimanual uterine compression. Make sure that everyone can see.
Internal
Internal bimanual
bimanual compression
compression
•
Follow steps for internal bimanual
uterine compression as listed in
the Reference Manual. Ask
participants to refer to the
Reference Manual and follow
along as you provide the
demonstration.
•
Ask for questions before
proceeding. Repeat any steps that
may be unclear.
•
Give participants time to practice
internal bimanual uterine
compression on the obstetric
model.
AT3-20
AT3-20
Managing
Managing complications
complications in
inpregnancy
pregnancy
and
and childbirth.
childbirth. W
WHO.
HO. 2003
2003
Facilitator’s Guide
Activity: Demonstration and return demonstration of aortic compression.
Notes to the facilitator:
•
•
Ask participants to stand
around the model to
observe the
demonstration of aortic
compression. Make sure
that everyone can see.
Follow steps for aortic
compression as listed in
the Reference Manual.
Ask participants to refer to
the Reference Manual
and follow along as you
provide the
demonstration.
Aortic
Aortic compression
compression
AT3-21
AT3-21
Managing
Managing complications
complications in
inpregnancy
pregnancy
and
andchildbirth.
childbirth. WHO.
WHO. 2003
2003
•
Ask for questions before
proceeding. Repeat any
steps that may be
unclear.
•
Give participants time to practice aortic compression on the obstetric model.
Flipchart / Overhead / PowerPoint slide 22
Time: 20 min
Activity: Small group work to review management of genital tears, retained placental
fragments, retained placenta.
Objective: Describe the immediate medical management of the woman whose uterus does
not contract adequately.
Notes to the facilitator:
Small
Small Group
Group Work
Work
AT3-22
AT3-22
•• Group
Group 1:
1: Develop
Develop aa job
job aid
aid that
that describes
describes
management
management of
of genital
genital tract
tract tears
tears (refer
(refer to
to
Additional
Additional Topic
Topic 33 in
in the
the Reference
Reference Manual).
Manual).
•• Group
Group 2:
2: Develop
Develop aa job
job aid
aid that
that describes
describes
management
management of
of retained
retained placenta
placenta (refer
(refer to
to
Additional
Additional Topic
Topic 33 in
in the
the Reference
Reference Manual).
Manual).
•• Group
Group 3:
3: Develop
Develop aa job
job aid
aid that
that describes
describes
management
management of
of retained
retained placental
placental fragments
fragments
(refer
(refer to
to Additional
Additional Topic
Topic 33 in
in the
the Reference
Reference
Manual).
Manual).
•
Divide the group in three smaller
groups. Assign each group the task
of preparing a job aid that
describes management of genital
tears (group 1), management of
retained placenta (group 2),
management of retained placental
fragments (group 3).
•
Give each group 10 minutes to
work on their task.
•
Circulate around the room and
assist participants as they work on
their task.
•
Each group should present their job
aid to the large group.
Facilitator’s Guide
Activity: Case study to review management of PPH.
Notes to the facilitator:
•
Divide participants into
groups of 3 to 4 people (try
to ensure that there is a
mix of cadres of providers in
each group).
•
Facilitate group work.
•
After 20 minutes, facilitate a
discussion about the
answers in a plenary.
Answers to the case study
are in the Facilitator’s
Guide and the
Participant’s Notebook.
Small
Small Group
Group Work
Work (Groups
(Groups of
of 3-4
3-4
people)
people)
AT3-30
AT3-30
••
Turn
Turn to
to classroom
classroom learning
learning activity
activity 33 for
for
Additional
Additional Topic
Topic 33 in
in the
the Participant’s
Participant’s
Notebook.
Notebook.
••
Carefully
Carefully read
read the
the case
case study
study and
and then
then
respond
to
the
questions
(refer
respond to the questions (refer to
to Additional
Additional
Topic
Topic 33 in
in the
the Reference
Reference Manual).
Manual).
20
minutes
20 minutes
••
Discuss
Discuss responses
responses in
in the
the plenary.
plenary.
10
10 minutes
minutes
Flipchart / Overhead / PowerPoint slide 31
Time: 60 min.
Activity: Clinical simulation to summarize key points in the session.
Clinical
Clinical simulation:
simulation: Management
Management of
of
vaginal
bleeding
after
childbirth
vaginal bleeding after childbirth
AT3-31
AT3-31
Notes to the facilitator:
• Refer to the Facilitator’s Guide
for instructions on how to
facilitate the clinical simulation.
•
Answers for the clinical simulation
are on the pages following
instructions for facilitating the
clinical simulation.
•
The questions, without the
answers, are located in the
Participant’s Notebook.
Facilitator’s Guide
Flipchart / Overhead / PowerPoint slide 32
Notes to the facilitator:
•
Encourage participants to work on learning activities found in the Participant’s
Notebook for Additional Topic 3.
•
Participants may work individually or in groups on the learning activities during
breaks, in the evening, or in the clinical area when there are no clients.
•
Participants may correct their learning activities by referring to suggested answers
found in the Participant’s Notebook. Facilitators should make themselves available
to work with the participants to review answers for learning activities.
Learning activities
AT3-32
• Please complete learning activities found in
the Participant’s Notebook for Additional
Topic 3
• You may work individually or in groups on
the learning activities during breaks, in the
evening, or in the clinical area when there
are no clients
• You may correct your answers individually or
with another participant or the facilitator.
• See a facilitator if you have questions.
Uterine atony:
uterus soft and relaxed
Treat for whole retained placenta
If whole placenta still retained
■ Manual removal with prophylactic antibiotics
Treat for retained placenta fragments
If bleeding continues
■ Manage as uterine atony
Lower genital tract trauma:
excessive bleeding or shock
contracted uterus
Treat for lower genital tract trauma
■ Repair of tears
■ Evacuation and repair of haematoma
If bleeding continues
■ Tranexamic acid
Uterine rupture or dehiscence:
excessive bleeding or shock
Treat for uterine rupture or dehiscence
■ Laparotomy for primary repair of uterus
■ Hysterectomy if repair fails
If bleeding continues
■ Tranexamic acid
Uterine inversion: uterine
fundus not felt abdominally or
visible in vagina
Treat for uterine inversion
■ Immediate manual replacement
■ Hydrostatic correction
■ Manual reverse inversion
(use general anaesthesia or wait for effect
of any uterotonic to wear off )
Clotting disorder:
bleeding in the absence of
above conditions
Treat for clotting disorder
■ Treat as necessary with blood products
Placenta not delivered
Placenta delivered incomplete
Be ready at all times to transfer
to a higher-level facility if the
patient is not responding to the
treatment or a treatment cannot
be administered at your facility.
Start intravenous oxytocin infusion
and consider:
• uterine massage;
• bimanual uterine compression;
• external aortic compression; and
• balloon or condom tamponade.
Transfer with ongoing intravenous
uterotonic infusion. Accompanying
attendant should rub the woman’s
abdomen continuously and, if
necessary, apply mechanical
compression.
Oxytocin – treatment of choice
■ Oxytocin
■ Controlled cord traction
■ Intraumbilical vein injection (if no bleeding)
■ Oxytocin
■ Manual exploration to remove fragments
■ Gentle curettage or aspiration
Ergometrine – if oxytocin is unavailable or bleeding continues despite
oxytocin
If laparotomy correction not successful
■ Hysterectomy
Prostaglandins – if oxytocin or ergometrine are unavailable or bleeding
continues despite oxytocin and ergometrine
Tranexamic acid
• 20–40 IU in 1 litre of intravenous fluid at 60 drops per minute, and 10 IU intramuscularly
• Continue oxytocin infusion (20 IU in 1 litre of
intravenous fluid at 40 drops per minute) until
haemorrhage stops
Section II: The following are laminated and displayed in a common area that is readily
accessible to physicians, nurse midwives, nurses, and other staff who might need the
information:
____WAPC “Algorithm for Postpartum Hemorrhage”
____WAPC list of “Uterotonic Agents for Postpartum Hemorrhage”
____Diagram of the B-Lynch compression suture technique
Oxytocin for Postpartum
Hemorrhage Protocol
revised October 2008
Preamble
One of the associated risks associated with childbirth is a postpartum hemorrhage. In the
out-of-hospital setting, early intervention to manage a significant and ongoing hemorrhage
can prevent further blood loss. Oxytocin helps contract uterine smooth muscle and
minimizes further uterine blood loss.
Requirements
1. Fully licensed Technician-Paramedic.
2. Certification in postpartum hemorrhage protocol by the Medical Director.
3. Certification in administration of intramuscular (IM) medication by the Medical Director.
Indications
1. Patients at greater than 20 weeks gestation who have delivered a newborn in the outofhospital environment.
and
2. Patients experiencing postpartum hemorrhage of greater than 500 ml blood.
Contraindications
1. Patient has not completed delivery of fetus(es).
2. Patient is less than 20 weeks gestation.
Oxytocin for Postpartum Hemorrhage Protocol
Drug Doses and Frequencies
oxytocin
IM: 10 IU after the newborn has delivered
IV: in the event of ongoing with significant blood loss, an additional 40 IU
can be added to each 1000 ml normal saline and infused based on the
severity of hemorrhage and patient response
Procedure
1. Perform patient assessment and record vital signs.
2. Assess that patient meets criteria for this protocol.
3. Ensure there are no contraindications to use of this protocol.
4. Initiate basic life support treatment measures, including supplemental oxygen.
- these take precedence over management using this protocol
5. Initiate an intravenous line with normal saline
- add oxytocin to the intravenous bag
- infuse at a rate based on severity of hemorrhage and patient condition
6. Manage the hemorrhage as per appropriate guideline or protocol.
7. While basic life support treatment measures and intravenous line are being initiated,
and hemorrhage is being controlled, obtain a focused obstetrical history. Include the
following details:
· antenatal care
· expected delivery date
· history of current pregnancy (including results of any ultrasounds)
· history of prior pregnancies (including history of previous difficulties)
8. If baby (last baby), but not placenta, has delivered:
· provide appropriate care for mother and newborn
· give mother oxytocin IM
· assist with delivery of placenta
· manage complications, if possible, as per appropriate guideline or
protocol
· initiate transport to hospital
Oxytocin for Postpartum Hemorrhage Protocol
3
9. If the baby (last baby) and placenta have delivered:
· provide appropriate care for mother and newborn
· give mother oxytocin IM if not already done as part of step 8
· manage complications, if possible, as per appropriate guideline or
protocol
· initiate transport to hospital
10.If possible, encourage mother to empty her bladder.
11.Massage the uterine fundus to promote uterine contraction and lessen the severity of
the hemorrhage.
12.Repeat assessment, including vital signs, level of consciousness, oxygen saturation,
and effect of oxytocin.
Documentation Requirements
The following information must be documented on the patient care report form:
1. Patient’s presenting signs and symptoms, including vital signs.
2. Indications for protocol use.
3. Details of patient’s obstetrical history and current delivery.
4. Dose, route, and time for each oxytocin dose used, and resulting clinical effects.
5. Repeat assessment and vital signs, as indicated.
6. Changes from baseline, if any, that occur during treatment or transport.
7. Signature and license number of EMS personnel performing any transfer of function skills.
Certification Requirements
1. Attend in-depth classes and lectures on obstetrics and obstetrical emergencies.
2. Demonstrate an understanding of the pharmacology, mechanism of action, and
potential side effects of oxytocin.
3. Do an acceptable clinical rotation on a labour and delivery ward.
Oxytocin for Postpartum Hemorrhage Protocol
4
4. Pass a written examination.
5. Pass practical scenarios incorporating variations of the oxytocin – postpartum
hemorrhage protocol.
6. Certification is by the Medical Director.
Recertification Requirements
1. Review class and recertification is done every 12 months.
2. A record will be kept to document all cases where this protocol is used.
Decertification
1. Decertification is at the discretion of the Medical Director or the Provincial Medical
Director, Emergency Medical Services, Manitoba Health & Healthy Living.
Quality Assurance Requirements
1. Appropriate quality assurance policies must be in place. The Medical Director or
designate must review all instances where this protocol is used. As a minimum, the
following must be assessed:
i) appropriateness of implementation
ii) adherence to protocol
iii) any deviation from the protocol
iv) corrective measures taken, if indicated
2. Yearly statistics for protocol use compiled and forwarded to Emergency Medical
Services, Manitoba Health & Healthy Living.