Enzyme Kinetics - Andrew.cmu.edu
advertisement
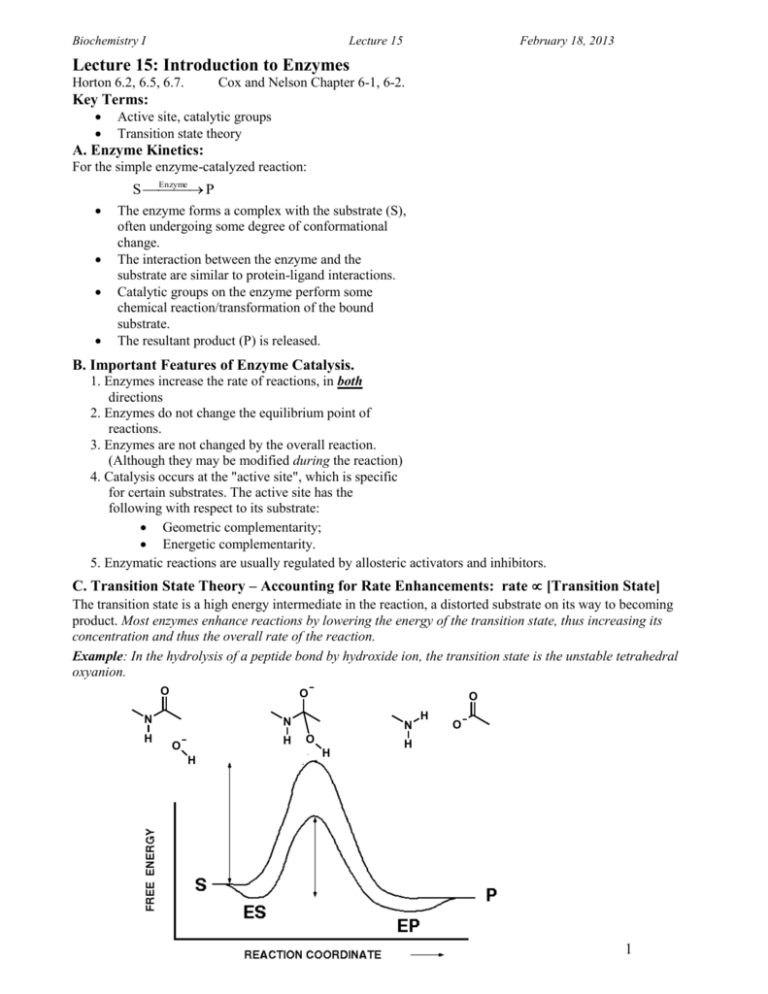
Biochemistry I Lecture 15 February 18, 2013 Lecture 15: Introduction to Enzymes Horton 6.2, 6.5, 6.7. Cox and Nelson Chapter 6-1, 6-2. Key Terms: Active site, catalytic groups Transition state theory A. Enzyme Kinetics: For the simple enzyme-catalyzed reaction: Enzyme S P The enzyme forms a complex with the substrate (S), often undergoing some degree of conformational change. The interaction between the enzyme and the substrate are similar to protein-ligand interactions. Catalytic groups on the enzyme perform some chemical reaction/transformation of the bound substrate. The resultant product (P) is released. B. Important Features of Enzyme Catalysis. 1. Enzymes increase the rate of reactions, in both directions 2. Enzymes do not change the equilibrium point of reactions. 3. Enzymes are not changed by the overall reaction. (Although they may be modified during the reaction) 4. Catalysis occurs at the "active site", which is specific for certain substrates. The active site has the following with respect to its substrate: Geometric complementarity; Energetic complementarity. 5. Enzymatic reactions are usually regulated by allosteric activators and inhibitors. C. Transition State Theory – Accounting for Rate Enhancements: rate [Transition State] The transition state is a high energy intermediate in the reaction, a distorted substrate on its way to becoming product. Most enzymes enhance reactions by lowering the energy of the transition state, thus increasing its concentration and thus the overall rate of the reaction. Example: In the hydrolysis of a peptide bond by hydroxide ion, the transition state is the unstable tetrahedral oxyanion. O O N N H H O H O N O H H O H 1 Biochemistry I Lecture 16 February 18, 2013 The transition state of the enzyme substrate complex is stabilized in many ways: 1. The enzyme may provide a number of functional groups to aid in catalysis. Since these groups are positioned in well defined locations, the entropy of bringing these groups into the catalytic site is substantially less than having these functional groups freely diffuse in solution. This entropy change contributes to G‡ via the relationship Go = -TSo. In this example, the three amino acids (Asp, His, and Ser) are involved in cleaving a peptide bond, converting the substrate (a peptide) to product via the transition state “X” Non-enzyme Reaction (same mechanism) Enzyme Catalysed Reaction Ser His S Asp His Ser X Asp His Ser S Asp His Ser X Asp Don't underestimate the effect of Entropy: O O H3C H3C C O H3C Br + O C H3C O O H2 C C H2 C C O Br C O O O C O O Br O H2 C C H2 C C O O O + Br O O 2. The enzyme transition-state complex is stabilized by direct interactions between the enzyme and the transition state. This reduces the free energy of the transition state due to Ho (enthalphy). O O N N H H O N O H H ES 2 O EX H H EP O Biochemistry I Lecture 16 February 18, 2013 3. The enzyme may provide a different mechanism to catalyze the reaction, e.g. transport proteins that facilitate the movement of polar molecules across the non-polar lipid bilayer. D. Quantitative Measure of Rate Enhancement by Stabilization of the Transition State: The rate or velocity (v) of the reaction is directly proportional to the concentration of the transition state: The substrates (and products) are in equilibrium with the transition state. Uncatalyzed Reaction Enzyme Catalyzed reaction v [X ] ve [ EX ] S X ES EX [ EX ] [ ES ] [X ] [S ] v K EQ [ S ] e ve K EQ [ ES ] G RT ln K EQ e Ge RT ln K EQ K EQ e G / RT e K EQ e Ge / RT v e G / RT [ S ] ve e Ge / RT [ ES ] K EQ e K EQ ve e Ge / RT [ ES ] ( G Ge ) / RT ( G ) / RT e e v e G / RT [ S ] 3