Patients` opinion of Early phase drug trials advertisement
advertisement
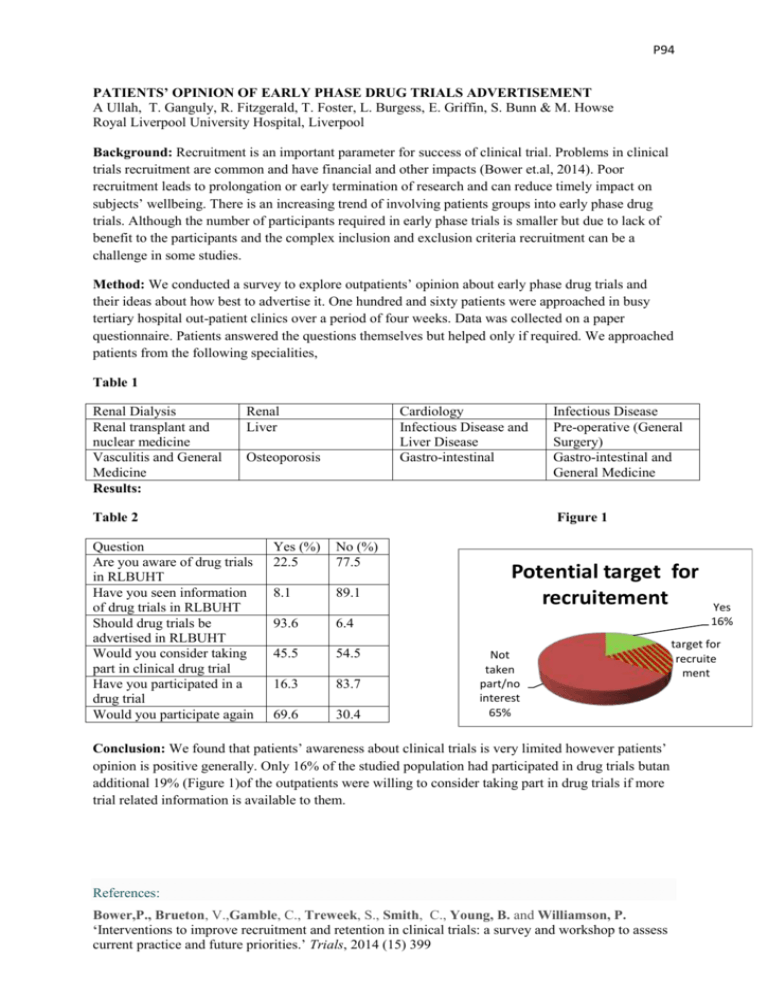
P94 PATIENTS’ OPINION OF EARLY PHASE DRUG TRIALS ADVERTISEMENT A Ullah, T. Ganguly, R. Fitzgerald, T. Foster, L. Burgess, E. Griffin, S. Bunn & M. Howse Royal Liverpool University Hospital, Liverpool Background: Recruitment is an important parameter for success of clinical trial. Problems in clinical trials recruitment are common and have financial and other impacts (Bower et.al, 2014). Poor recruitment leads to prolongation or early termination of research and can reduce timely impact on subjects’ wellbeing. There is an increasing trend of involving patients groups into early phase drug trials. Although the number of participants required in early phase trials is smaller but due to lack of benefit to the participants and the complex inclusion and exclusion criteria recruitment can be a challenge in some studies. Method: We conducted a survey to explore outpatients’ opinion about early phase drug trials and their ideas about how best to advertise it. One hundred and sixty patients were approached in busy tertiary hospital out-patient clinics over a period of four weeks. Data was collected on a paper questionnaire. Patients answered the questions themselves but helped only if required. We approached patients from the following specialities, Table 1 Renal Dialysis Renal transplant and nuclear medicine Vasculitis and General Medicine Results: Renal Liver Cardiology Infectious Disease and Liver Disease Gastro-intestinal Osteoporosis Table 2 Question Are you aware of drug trials in RLBUHT Have you seen information of drug trials in RLBUHT Should drug trials be advertised in RLBUHT Would you consider taking part in clinical drug trial Have you participated in a drug trial Would you participate again Infectious Disease Pre-operative (General Surgery) Gastro-intestinal and General Medicine Figure 1 Yes (%) 22.5 No (%) 77.5 8.1 89.1 93.6 6.4 45.5 54.5 16.3 83.7 69.6 30.4 Potential target for recruitement Not taken part/no interest 65% Conclusion: We found that patients’ awareness about clinical trials is very limited however patients’ opinion is positive generally. Only 16% of the studied population had participated in drug trials butan additional 19% (Figure 1)of the outpatients were willing to consider taking part in drug trials if more trial related information is available to them. References: Bower,P., Brueton, V.,Gamble, C., Treweek, S., Smith, C., Young, B. and Williamson, P. ‘Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities.’ Trials, 2014 (15) 399 Yes 16% target for recruite ment







