Chemotherapy-Induced Nausea and Vomiting
advertisement

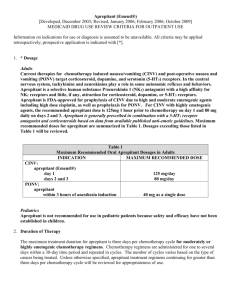
1 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Chemotherapy-Induced Nausea and Vomiting Anne M. Hendricks Ferris State University 2 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Abstract Inconsistent usage of antiemetic therapies during chemotherapy treatment led me to research the latest evidence, develop charts for staff, and tracking mechanisms for inconsistencies. I utilized information from our Oncology Pharmacist (Joanne McGurn) and several evidence-based research articles to formulate charts of the recommended treatments and dosages for high, moderate and low emetogenic chemotherapy (Appendixes A,B,C). I placed the charts in our medication room in a binder, and sent copies to all seven Oncologists that we serve. I developed a chart that measures each time a correction is required for specific physicians (Appendix D). I developed another chart to measure patient subjective perceptions of nausea and vomiting (Appendix E). Severity of nausea symptoms was measured by number 1-4, with 1=minor nausea/vomiting, 2=more severe nausea/vomiting which influences activity slightly, 3= very activity limiting nausea/vomiting, 4=debilitating nausea/vomiting. Continued research of monthly data and collaboration with physician/pharmacist will ensure best patient outcome through continuity of care. 3 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Chemotherapy-Induced Nausea and Vomiting I work in the Infusion Clinic at Munson Medical Center and I have a National Oncology Certification. I have noticed some inconsistency with the usage of antiemetics amongst physicians treating our cancer patients. Our Oncologists tend to rely on nursing or pharmacy to catch/correct antiemetic errors. Because of the dynamic nature of cancer and chemotherapy treatments, and the different Oncologists’ preferences for antiemetics, it is difficult for the nurse to know if the antiemetic chosen is appropriate. There are times when the most appropriate medication is missed and the patient suffers. I wanted to develop a way to promote patient comfort by ensuring proper, consistent antiemetic usage and prevent chemotherapy-induced nausea and vomiting. My research is from the most current antiemetic studies which I have summarized. My first research was a study performed by Warr and Oliver (2005) regarding Neurokinin-1 (NK1) receptor antagonist (Aprepitant). Warr and Oliver’s (2005) study came from randomized controlled trials, one systematic review, and one meta-analysis; and the funding came from Cancer Care Ontario and Ontario Ministry of Health and Long-Term Care. The subjects were > 16 years old, with a Karnofsky performance Index > 60, which meant that they were at least able to perform normal activities without much assist and able to live at home (Warr & Oliver, 2005). The subjects were stratified by gender, had solid tumors, and were to be receiving high dose Cisplatin, chemotherapy with a high emetogenic index (Warr & Olviver,2005). Differing doses of Aprepitant from 40 mg- 400 mg were compared, with 125 mg being the most efficacious by showing 90% receptor occupancy on day one of treatment (Warr & Oliver,2005). The five trials revealed a significant increase, 12.5 %-20 %, in complete protection 4 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING from nausea and vomiting with Aprepitant (Warr & Oliver, 2005). Another study by Kris et al.(2006) published in the American Society of Clinical Oncology analyzed antiemetic data found in the Medline Database and Cochrane Library. Update committee members contributed articles and reviewed collected material on published, randomized, controlled trials in phase II and III (Kris et al.,2006). The focus was on Aprepitant, (Neurokinin-1 [NK1] receptor antagonists), 5-hydroxytryptamine(3) (5-HT3) receptor antagonists and Dexamethasone in high-dose Cisplatin therapy (Kris et al.,2006). The study defined chemotherapy emetic risks as High: 90% chance, Moderate: 30%-90%, Low: 10%-30%, and Minimal: <10% (Kris et al.,2006). The study also gave recommendations for antiemetic regimens based on the chemotherapy emetic potential. High: 5-HT3, Dexamethasone and Aprepitant pre-chemo day 1, Aprepitant and Dexamethasone day 2 and day 3 (Kris et al.,2006). Moderate: 5-HT3, Dexamethasone and Aprepitant day 1 and 5-HT3 or Dexamethasone day 2 and day 3 and Low: Dexamethasone pre, no delayed prevention routinely (Kris et al.,2006). Kris et al.(2006) also noted that combination chemotherapy regimens require treating the highest emetic risk and multiple-consecutive days of chemotherapy require treating the highest emetic risk each day of therapy and 2 days post treatment. The study by Kris et al.,(2006) did offer one area of concern regarding the NK1 decreasing clearance of certain chemotherapies via cytochrome P450 (CYP3A4), leading to potential toxicity, but stressed that there is not enough evidence to support this at this time. The third source of information is from the National Comprehensive Cancer Network (NCCN) guidelines (2008) found at http://www.nccn.org, which, according to J. McGurn, PhD, are the guidelines followed by our Oncology pharmacy (personal communication, October 29,2008). The NCCN uses a multidisciplinary panel to review the latest treatments, support 5 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING clinical trials, and update its guidelines with the most current and comprehensive information available (NCCN, October 2008). For the purpose of this paper, I mention only the latest findings from the website which are in addition to my research defined above. More information is available at the website (http://www.nccn.org). According to the NCCN (2008) guidelines a new FDA approved drug for high and moderate emetogenic chemotherapy was introduced: Fosaprepitant Dimeglumine 115 mg intravenous administered over 15 minutes can replace Aprepitant 125 mg orally on day 1 of treatment, followed by Aprepitant oral 80 mg day 2 and day 3. Careful monitoring of drugs metabolized by cytochrome P450 and enzyme 3A4 (CYP3A4) is suggested when using the drug Aprepitant because it can alter metabolism (NCCN,2008,MS-4). The NCCN (2008) mention another drug Palnosetron, 5-HT3 antagonist with 100-fold higher binding affinity compared to other 5-HT3, which has a half-life of approximately 40 hours and has shown superiority in delayed emesis. The NCCN does state that further research is being performed on safety of this drug, and I know that neither Palnosetron nor Fosaprepitant Dimeglumine are in our hospital formulary as of yet (personal communication J.McGurn, October 30,2008). The rest of the antiemetic protocols are as my prior research showed. How I conducted my research: I began in Medline Database under “antiemetics” and I found several articles but I narrowed my search by choosing the most recent. I then called our Oncology Pharmacist (J. McGurn) to find out what the current guidelines were for the hospital. J.McGurn said that the pharmacists utilize http://www.nccn.org website then go under “Clinical Practice Guidelines in Oncology”, then under “Guidelines for Supportive Care”, then under “Antiemesis”, and finally “V.3.2008”. I compiled a list of the drugs currently on formulary, both chemo and antiemetic, to comprise charts (Appendix A,B,C) which I placed in the medication 6 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING room in an “important information” folder. The charts define which of the drugs that we use in the clinic as High, Moderate or Low risk and have the appropriate antiemetic therapy available below each category. I am hoping that this will be an easy reference tool to prevent antiemetic errors. If a nurse finds a discrepancy she (all females in our clinic) is to alert the Oncology Pharmacist who can then make a corrected order or refer the nurse to the appropriate Oncologist. I am also going to send copies of the Appendix A-C to each of the Oncologists. I hope to measure our success by keeping a check sheet with dates for each calendar month and each of our Oncologists’ names, so that if a nurse finds an error in antiemetic that requires correction by physician or pharmacist they mark the appropriate physician corresponding with appropriate date of occurrence (Appendix D). Comparison studies for each calendar month will be kept for reference. Another measure (Appendix E) will be subjective information gathered from the patient. The nurses are to record nausea and vomiting with a patient-defined number of severity: 1=mild nausea, 2=Slight nausea/vomiting: interferes somewhat with activity, 3=Severe nausea/vomiting: interferes greatly with activity, 4=Debilitating nausea/vomiting: unable to care for self. Patient name sticker will be placed, which also has physician name, in the date correlating with symptoms. This chart can be used in many ways, to monitor the physician, the patient, and the chemotherapy for any patterns. The information can be utilized by the nurses, physicians and pharmacists. The nursing theory that I felt most related to my project was Kolcaba’s Theory of Comfort which, according to Alligood and Tomey (2006), states that health care needs are needs of comfort which can be measured by nursing interventions specific to the patient. According to Herrstedt (2007) patients consider nausea and vomiting some of the worst side-effects of chemo. 7 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Navari (2003) sites chemo-induced nausea and vomiting as a condition to “quality of life”. I feel that if nausea and vomiting can be controlled through consistent use of antiemetics then patients’ healthcare needs of comfort will be better met. 8 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING References Alligood,M.R.,&Tomey,A.M.(2006).Nursing Theorists and Their Work (6th ed.). Missouri: Mosby-Elsevier. Food and Drug Administration. (2008,October). RX Trials institute drug pipeline alert: AP Pharma gets mixed results. 6 (41). Retrieved October 23, 2008, from http://fdanews.com/newsletter/article?issueId=12025&articleId=111062 Herrstedt,J.(2007). Medical treatment of chemotherapy-induced nausea and vomiting. Ugeskr Laeger,169(9),799-805. Kris, M.G.,Hesketh,P.J.,Somerfield,M.R.,Fever,P.,Clark- Snow,R.,Koeller,J.M.,et al. (update 2006). Guideline for antiemetics in oncology. American Society of Clinical Oncology,24(18)1-16.[110references]. National Comprehensive Cancer Network. (2008,February 5). Clinical practice guidelines in oncology: Guidelines for supportive care-Antiemesis. (v.3 2008)[electronic version] retrieved October 30,2008, from http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf. Navari,R.M.(2003).Pathogenesis-based treatment of chemotherapy-induced nausea and vomiting—two new agents. Journal of Supportive Oncology, 1(2),89-103. Warr,D.& Oliver,T. (2005). The role of neurokinin-1 receptor antagonists in the prevention of emesis due to high-dose cisplatin. Cancer Care Ontario (2005, April 5).p.27 (Evidencebased series no.12-4).[20 references]. 9 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Appendix A Antiemetic Chart High Emetogenic: (90 % likelihood of emesis) Adriamycin and Cytoxan Cisplatin Cytoxan >1500mg/m2 DTIC Antiemetic regimen: Aprepitant 125 mg PO day 1, 80mg PO day 2 and day3 OR Fosaprepitant dimeglumine 115mg IV day 1, aprepitant 80mg PO day 2 and day3 AND Dexamethasone 12 mg PO/IV day1, 8 mg PO/IV days 2-4 AND 5-HT3 antagonist Ondansetron 16-24 mg PO or 8-12 mg (max. 32 mg) IV day 1 OR Granisetron 2 mg PO or 1 mg PO BID or max 1mg IV day 1 OR Palososetron 0.25 mg IV day 1 AND prn. Lorazepam 0.5-2 mg PO/IV/SL every 4-6 hours days 1-4 10 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Appendix B Antiemetic Chart Moderate emetogenic: (30-90% frequency of emesis) Amifostine >300mg/m2 Carboplatin Cisplatin <50 mg/m2 Cytoxan < 1500mg/m2 or PO Doxorubicin, Daunorubicin, Epirubicin, Idarubicin Etoposide Ifosfamide Irinotecan Methotrexate Oxaliplatin >75mg/m2 Antiemetic regimen: Aprepitant 125 mg PO day 1, 80 mg PO day 2 and day 3 OR Fosaprepitant dimeglumine 115 mg IV day 1 with Aprepitant 80 mg PO day 2 and day 3 Dexamethasone 12 mg PO/IV day 1, 8 mg PO/IV daily Or 4mg BID day 2-4 5-HT3 antagonist: Palonosetron 0.25 mg IV day 1 PO or 1 mg PO BID day 1 Or Granisetron 1-2mg Or Ondansetron 16-24 mg PO or max 32 mg/day IV 5-HT3 can be added day 2 and day 3. Lorazepam 0.5 mg-2 mg PO/IV/SL every 4-6 hours as needed 11 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Appendix C Antiemetic Chart Low Emetogenic: (10-30% frquency of emesis) Amifostine < 300mg Capecitabine Cytarabine 100-2--mg/m2 Docetaxal Doxorubicin (liposomal) Etoposide 5-Flourouracil Gemcitabine Methotrexate 50-250mg/m2 Mitomycin Mitoxantrone Paclitaxel and Paclitaxel-albumin Pemetrexed Topotecan Antiemetic regimen: Dexamethasone 12 mg PO/IV daily OR Prochlorperazine 10 mg PO/IV every 4-6hours OR Diphenhydramine 25-50mg PO/IV every 4-6 hours OR Lorazepam 0.5-2mg PO/IV every 4-6 hours if needed 12 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Appendix D Physician: Dr. Gordon Dr. Hughes Dr. Kohler Dr. Kosinski Dr. Michelin Dr. Ramsdell Dr. Schwert Dates the antiemetic order had to be corrected 13 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Appendix E Mon Tue Wed Thu November 2008 Fri Place patient identification On the correct date with number 3 4 5 6 7 corresponding to Severity of nausea and vomiting: 1=Mild Nausea 10 11 12 13 14 2=Slight Influence on Activity 3=Very Activity Limiting Nausea and Vomiting 17 18 19 20 21 24 25 26 27 28 4=Debilitating Nausea and Vomiting 14 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Addendum In the process of doing my research I remembered that one of our patients had neurotoxicity thought related to chemotherapy, but was rather unusual. The patient didn’t have a problem in the first treatment cycle, but developed a problem at the end of the second treatment cycle. The patient became noticeably slower to process. The third cycle of treatment the patient had definite processing deficits and had a facial twitch. The patient was stopped from receiving the chemotherapy. This is a 44 year old man with a four year old daughter. I couldn’t stop thinking about the patient, and wondering what was the difference between the first and the subsequent rounds? I found an article describing a possible link between the antiemetic and chemotherapy which may cause neurotoxicity (Howell, Szabatura, Jatfield, and Nesbit, 2008). I remembered that in the first cycle of chemotherapy the patient was not on Aprepitant because it was not prescribed, but, because he had severe nausea and vomiting, he was placed on Aprepitant for the subsequent treatments. That was the answer to the difference between cycles question. I then remembered that Aprepitant is the NK1 that can interfere with metabolism of some medications (NCCN,2008). I further searched and found an article stating that the chemotherapy the patient was receiving (Ifofsamide) may be one of the drugs that Aprepitant interferes with (Howell,et al.,2008). Ifofsamide is “ metabolized by the cytochrome P450 system to its active form, …a potential side effect is neurotoxicity, often manifesting as confusion, hallucination, or seizure.” (Howell,et al., 2008).I shared the information with Joanne McGurn, Oncology pharmacist, who has since shared the information with the physician and the U of M Sarcoma Clinic, who are also involved in care. Last week the patient was able to receive an off formulary drug, Palonosetron, that was the newest 5-HT3 agonists mentioned by NCCN 2008. The patient has done well so far, and will continue treatment. I strongly believe that this is 15 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING evidence-based practice at its finest. My questioning and researching the latest evidence led to an application in my own practice. This did change how the patient was treated in the future and was shared with UofM as well, policies regarding this regimen were changed in our hospital to avoid future problems. The Oncologist called me personally to thank me and said, “Good catch”. I really wanted to share this with you, I feel like this process has really helped me grow considerably in my profession and, best of all, has possibly helped a patient. 16 Running head: CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING References Howell,J.E.,Szabatura,A.H.,Hatfield,S.A., & Nesbit,S.A.(2008). Characterization of the occurrence of ifosfamide-induced neurotoxicity with concomitant aprepitant. Journal of Oncology Pharmacy Practice,14(3),157-162. National Comprehensive Cancer Network. (2008,February 5). Clinical practice guidelines in oncology: Guidelines for supportive care-Antiemesis. (v.3 2008)[electronic version] retrieved October 30,2008, from http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf.
![[Physician Letterhead] [Select Today`s Date] . [Name of Health](http://s3.studylib.net/store/data/006995683_1-fc7d457c4956a00b3a5595efa89b67b0-300x300.png)






![Questionnaire used in the study Demographics GENDER: M [ ] F](http://s3.studylib.net/store/data/006712173_1-21c851410b04058d524e1b79e54e32b0-300x300.png)