HL7 Allergy and Intolerance Minutes HL7 Patient Care Work Group
advertisement

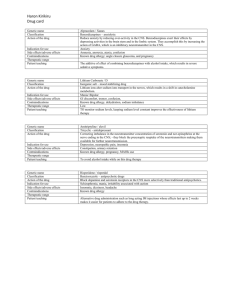
HL7 Patient Care Work Group Allergy/Intolerance/Adverse Reaction Topic Sub-Group Meeting Minutes Date: October 29, 2014 Phone Number: +1 770-657-9270 Participant Passcode: 943377 WEBEX: 197 520 889 Co-Chairs: Stephen Chu/Elaine Ayres Name Elaine Ayres Stephen Chu Kelly Verdin Rob Hausam Matt Graham Emma Jones Jay Lyle Russ Leftwich Scribe: Elaine Ayres Present on October 29, 2014 X X X X X X X X Agenda for October 29, 2014 1. Approve minutes of meetings: 2. Discussion of proposed AdverseReactionRisk FHIR Resource a. Link to OpenEHR comments: http://wiki.hl7.org/images/6/6d/OpenEHR-FHIR-AdverseReactionRiskreview-summary_2014-10-09.pdf 3. Continue work with C-CDA harmonization 4. Other items? 5. Next meeting – Wednesday, November 12 Minutes for October 15: Move: Russ/Stephen Abstain: 1 Negative: 0 Approve: 6 The group discussed problem status vs. the allergy problem and appropriate concepts for the value sets. It was noted that often an allergy list includes the morphologic presentation (as opposed to the condition). Therefore a list might be rash, swelling, hives etc. The group discussed what should go on the list. It was also noted that these terms should be crossed-mapped with ICD-10. The FHIM group (Federal Health Information Management) did a study of system allergy lists. The study included allergy lists from the VA, Intermountain Health and Kaiser Permanente and examined the frequency of reaction/manifestation terms. (This is currently unpublished work but will be used to drive a value set in VSAC for reaction terms). The top manifestation terms across systems in descending order was: RASH NAUSEA AND VOMITING URTICARIA ANAPHYLAXIS HL7 Allergy and Intolerance Minutes MUSCLE PAIN DYSPNEA NAUSEA COUGH SWELLING DIARRHEA PRURITUS If a reaction has two manifestations it may lead to two different conditions. So for example, sulfa drugs may cause nausea and vomiting and urticaria indicating the need to include two different conditions. For an allergy problem – the problem is inactive until challenged. However the risk is always active. The group then discussed the list of substance types – and agreed that the synthetic substance could be removed. FHIR resources for adverse reaction and allergy and intolerance and comparative analysis with the proposed adverse reaction risk: The group discussed the new combined resource as proposed by FHIR which combines the reaction and the condition. The new combined resource is called the “AdverseReactionRisk” and the UML model is as follows: The group discussed if there was a need to represent an adverse reaction without the representation of the condition. Russ noted that 80% of adverse reactions are situational and not the propensity to react related to an individual. Rob noted that when there is an event, it may not be known what the causative substance is until later. It was also noted that 50% of hives had no cause. 2|P a g e HL7 Allergy and Intolerance Minutes The group then discussed if an adverse reaction should be changed to an adverse event. In risk management an adverse event could be anything including a fall, an incorrect drug and may or may not have an effect. It was noted that the construct of a FHIR resource for adverse reaction will help clarify the discussion. C-CDA Harmonization – postponed. Problem Observation – proposed terms Allergy Status Observation – proposed terms Proposed terms for Table 117 – substance class Agenda for November 12 , 2014 1. Summary of Substances Summit – November 5-7, 2014 2. Adverse reaction resource 3. C-CDA harmonization. 3|P a g e