File
advertisement

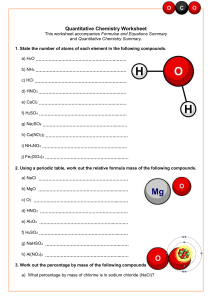
______________ ______________ ______________ Introduction Atoms are not created or destroyed during a chemical reaction. Scientists know that there must be the same number of atoms on each side of the equation. To balance the chemical equation, you must add coefficients in front of the chemical formulas in the equation. You cannot add or change subscripts!! Procedures 1. Determine the number of atoms for each element. 2. Pick an element that is not equal on both sides of the equation. 3. Add a coefficient (the large number to the left of the formula) in front of the formula with that element and adjust your counts. 4. Continue adding coefficients until you get the same number of atoms of each element on each side. 5. If you do not need to add a coefficient, place a “1” in the box. Ca + O → CaO N 2 Cu O + C →Cu + CO 2 H 2 + O 2 → H O 2 2 2 + H → NH 2 3 Na + MgF →NaF +Mg 2 Mg + O → MgO 2 Na + Al2O3 P4 C + + O2 → → Al Na2O + O2 O2 → P4O10 H2 → C2H4 Bonus: C3H5(NO3)3 → CO2 + N2 + H2O + O2 Balancing Chemical Equations Name:_________________________________Date__________Period________ The law of conservation of mass states the in a chemical reaction mass cannot be created or destroyed, it can be rearranged or turned into energy. The following chemical reactions are not balanced. Study the examples below and then balance the rest of the equations to show that mass was conserved. Ex—HgO → Hg + O2 balanced it is: Ex – Mg + HCl → 2HgO→ 2Hg + O2 MgCl + H2 balanced is: 2Mg + 2HCl → 2MgCl + H2 Now balance these equations: 1. NaCl + AgNO3 → NaNO3 + AgCl 2. Zn + H2SO4 → ZnSO4 + H2 3. NaOH + HCl → NaCl + H2O 4. Al2(SO4)3 + Ca(OH)2 → 5. H2O2 → H2 + O2 6. Cl2 + NaBr → 7. Zn + CuSO4 → 8. KClO3 → KCl + O2 9. H2O + Fe → 10. Ca(OH)2 + HNO3 → 11. Na2O + CO2 → 12. H2 + N2 → NH3 13. HgO + Cl2 Al(OH)3 + CaSO4 NaCl + Br2 ZnSO4 + Cu Fe2O3 + H2 → Ca(NO3)2 + H2O Na2CO3 HgCl + O2 14. Na + Br2 → 15. Ca(OH)2 + HNO3 → NaBr Ca(NO3)2 + H2O