Heat Transfer Article
advertisement

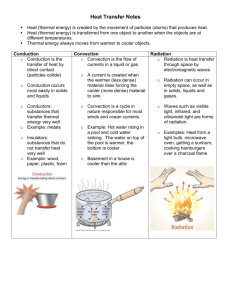
Heat and Heat Transfer What is heat? Heat is a form of energy that raises the temperature of an object. Heat is the amount of thermal energy entering or leaving an object due to a difference in temperature. Heat increases the movement of atoms and molecules in a substance. What is temperature? Temperature is a measure of how hot or cold something is, with respect to some standard scale as measured using a thermometer. Temperature is related to the average kinetic energy (or the energy of motion) of the individual atoms (or molecules) in a substance as they move and collide with one another. The hotter something is, the faster the atoms are moving, and the higher their kinetic energy. Heat Transfer If an object is surrounded by something at the same temperature, then it will remain at that temperature. On the other hand, if the object is surrounded by something at a lower temperature, then it will transfer some of its energy to the cooler material surrounding it, and the object will grow colder. If an object is surrounded by something at a higher temperature, some of the energy from its surroundings will be transferred to the object, and the surroundings will grow colder (unless that energy is replaced). The hotter, fast-moving atoms come into contact with the slow-moving atoms of the cooler substance. In these collisions, slower particles will tend to be sped up; faster particles will tend to be slowed down. As a result, the average energy of the particles in the cooler object will increase, raising its temperature, whereas the average energy of the particles in the hotter object will decrease, lowering its temperature. Heat (or thermal energy) flows from a hotter material to a cooler one. Heat flow continues until both objects reach the same temperature. Eventually, the temperature of the two objects will equalize, and there will be no more net flow of energy from one to the other. This situation is called thermal equilibrium. Conduction, Convection and Radiation There are three ways in which heat can be transferred: conduction, convection and radiation Conduction is thermal energy (or heat) moving within a material. If you touch a hot frying pan, its thermal energy will be transferred to your hand. You will feel the heat. Some materials conduct heat better than others. Water and land/soil will conduct heat to the water, air and land/soil around it. Convection is heat transport by material moving from one place to another. Hot material rises and cooler material sinks. Heating a pan of water on a stove or hotplate makes the water in the bottom of the pan hotter. The heated water expands. Hot water is less dense than cold water. So the hot water rises to the top of the pan, and the cooler, denser water at the top sinks to the bottom. This transfers heat from the bottom throughout the pan of water. (Lava lamps also illustrate convection or hot material rising, and cool material sinking.). In our atmosphere, warm air rises over warm land and water, as it does so it pushes the cooler air down through the process of convection. Radiation is heat energy carried by electromagnetic waves. Heat from the Sun is transferred by radiation through space to other objects in the Solar System, including the Earth. Objects in space (where there is no air) are heated by radiation on the side facing the Sun. Then, if there is no atmosphere (like on the Moon, or on asteroids), most of that heat is reflected and re-radiated back into space. The Earth is heated by radiation from the Sun during the day. Some of that heat radiates back into space at night. However, the Earth's atmosphere acts like a "blanket" and keeps much of the heat from escaping during the night. Clouds are a better "blanket" than air because clouds (which are made of droplets of liquid water) absorb more of the heat radiating from the Earth's surface into space. Read the article “Heat and Heat Transfer?” and answer the following questions: 1. What is heat? ____________________________________________________________________________________ 2. What happens to the speed of atoms and molecules of an object as it gets hotter? _____________________________ __________________________________________________________________________________________________ 3. What is temperature? ______________________________________________________________________________ 4. What is the relationship between temperature, and kinetic energy? _________________________________________ __________________________________________________________________________________________________ 5. What would happen if a container of cold water was surrounded by air that was warmer (higher temperature)? __________________________________________________________________________________________________ __________________________________________________________________________________________________ 6. What would happen if a container of warm water was surrounded by air that was colder (lower temperature)? __________________________________________________________________________________________________ __________________________________________________________________________________________________ 7. How does energy flow between two objects? ___________________________________________________________ 8. What is thermal equilibrium? ________________________________________________________________________ __________________________________________________________________________________________________ 9. Describe each of the three ways heat can transfer from one object to another: Conduction: _______________________________________________________________________________________ __________________________________________________________________________________________________ Example: ___________________________________________________________________________________ Convection: _______________________________________________________________________________________ __________________________________________________________________________________________________ Example: ___________________________________________________________________________________ Radiation: ________________________________________________________________________________________ __________________________________________________________________________________________________ Example: ___________________________________________________________________________________ 10. This reading had two main ideas about how hot materials affect colder materials and how hot materials behave with colder materials. What are those two main ideas? __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________