List of Abbreviations ACS Acute Coronary Syndromes C9AzPC 1
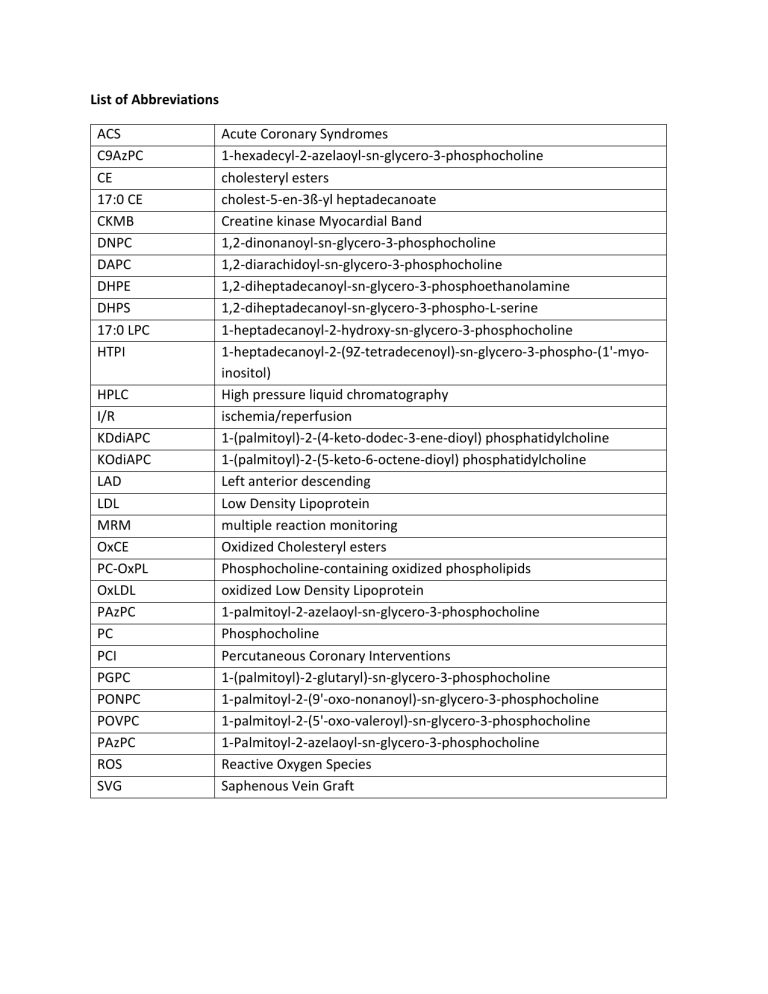
HPLC
I/R
KDdiAPC
KOdiAPC
LAD
LDL
MRM
OxCE
PC-OxPL
OxLDL
PAzPC
PC
PCI
PGPC
PONPC
POVPC
PAzPC
ROS
SVG
List of Abbreviations
ACS
C9AzPC
CE
17:0 CE
CKMB
DNPC
DAPC
DHPE
DHPS
17:0 LPC
HTPI
Acute Coronary Syndromes
1-hexadecyl-2-azelaoyl-sn-glycero-3-phosphocholine cholesteryl esters cholest-5-en-3ß-yl heptadecanoate
Creatine kinase Myocardial Band
1,2-dinonanoyl-sn-glycero-3-phosphocholine
1,2-diarachidoyl-sn-glycero-3-phosphocholine
1,2-diheptadecanoyl-sn-glycero-3-phosphoethanolamine
1,2-diheptadecanoyl-sn-glycero-3-phospho-L-serine
1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine
1-heptadecanoyl-2-(9Z-tetradecenoyl)-sn-glycero-3-phospho-(1'-myoinositol)
High pressure liquid chromatography ischemia/reperfusion
1-(palmitoyl)-2-(4-keto-dodec-3-ene-dioyl) phosphatidylcholine
1-(palmitoyl)-2-(5-keto-6-octene-dioyl) phosphatidylcholine
Left anterior descending
Low Density Lipoprotein multiple reaction monitoring
Oxidized Cholesteryl esters
Phosphocholine-containing oxidized phospholipids oxidized Low Density Lipoprotein
1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine
Phosphocholine
Percutaneous Coronary Interventions
1-(palmitoyl)-2-glutaryl)-sn-glycero-3-phosphocholine
1-palmitoyl-2-(9'-oxo-nonanoyl)-sn-glycero-3-phosphocholine
1-palmitoyl-2-(5'-oxo-valeroyl)-sn-glycero-3-phosphocholine
1-Palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine
Reactive Oxygen Species
Saphenous Vein Graft
METHODS
Materials
Synthetic standards 1,2-dinonanoyl-sn-glycero-3-phosphocholine (DNPC), 1,2diarachidoyl-sn-glycero-3-phosphocholine (DAPC), 1,2-diheptadecanoyl-sn-glycero-3phosphoethanolamine (DHPE), 1,2-diheptadecanoyl-sn-glycero-3-phospho-L-serine (DHPS), 1heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine ( 17:0 LPC), 1,2-diheptadecanoyl-snglycero-3-phospho-(1'-rac-glycerol) (DHPG), 1-heptadecanoyl-2-(9Z-tetradecenoyl)-sn-glycero-
3-phospho-(1'-myo-inositol) (HTPI), 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine
(POVPC), 1-palmitoyl-2-glutaroyl-sn -glycero-3-phosphocholine (PGPC), 1-palmitoyl-2-(9oxo)nonanoyl-sn-glycero-3-phosphocholine (PONPC) and cholest-5-en-3ß-yl heptadecanoate
(17:0 CE) were obtained from Avanti Polar Lipids (Alabaster, AL). 1-Palmitoyl-2-azelaoyl-snglycero-3-phosphocholine (PAzPC) and 1-palmitoyl-2-(9-oxo)nonanoyl-sn-glycero-3phosphocholine (PONPC), 1-(palmitoyl)-2-(5-keto-6-octene-dioyl)-3-phosphocholine (KOdiAPC),
1-palmitoyl-2-(4-keto-dodec-3-ene-dioyl)-sn-glycero-3-phosphocholine (KDdiAPC) were purchased from Cayman Chemicals (Ann Arbor, MI). PAPC, PLPC, SAPC, SLPC, SDHPC, PDHPC were oxidized as previously described (14,16). All solvents were HPLC grade.
Patients and sample collection
Twenty-four distal protection devices were obtained from 24 patients undergoing clinically indicated coronary and peripheral procedures that included stent placement in all cases: 16 patients underwent saphenous vein graft (SVG) interventions, of which 14 had stable symptoms undergoing elective angiography and 3 had an acute coronary syndrome (ACS) manifested by non ST-segment elevation myocardial infarction, 2 patients with renal artery stenosis and uncontrolled hypertension on maximal medical therapy, 3 patients with carotid disease with >80% stenosis without prior stroke or transient ischemic attack and 3 patients with superficial femoral artery (SFA) disease and lifestyle limiting claudication. None of these patients had peri-procedural events.
Material from 16 SVG filters was analyzed for OxPL and OxCE by LC-MS/MS, of which a subset of 7 filters were analyzed for ELISA measurement for the presence of apolipoprotein B-
100 and OxPL, as well as lipoprotein(a) and plasminogen, which are known to carry OxPL (1,2).
Four SVG filters were analyzed by LC-MS/MS for relative quantitation of specific OxCE and 4
SVG filters were analyzed immunohistochemically for the presence of OxPL. The collection of materials was approved by the UCSD Human Research Subjects Protection Program.
At the end of the procedure, the recovered filters from the distal protection devices were immediately cut off from the wire and the entire filter with the retained biological material was placed in ice-cold phosphate buffered solution containing EDTA/BHT (4µM/20µM) and the recovered plaque material was sonicated. The filter material was then lipid extracted by the Folch method and analyzed immediately by liquid chromatography, tandem mass spectrometry (LC-MS/MS). For total lipid extraction, 500 μl of filter material homogenates were transferred into a glass tube, 1.25 ml of ice-cold chloroform/methanol (2:1) containing internal phospholipid standards (9:0-9:0 PC, 20:0-20:0 PC, 14:1-17:0 PI, 17:0 LPC, 17:0-17:0 PS, 17:0-
17:0 PE, 17:0-17:0 PG) and neutral lipid standards (CE 17:0) were added and the tubes vortexed at a maximum speed for 30 seconds. Phase separation was achieved with addition of 1.85 mL chloroform and 450 μl of 0.2M KCl. After centrifugation, the lower organic phase was transferred into a fresh glass tube using a Pasteur pipette, and the organic phase was dried under argon and reconstituted in HPLC mobile phase prior to injection. Copper oxidized LDL
(OxLDL) was used as a comparator in some studies and prepared as previously described (3).
HPLC-MS/MS of PC-OxPL
For PC-OxPL analysis a normal phase, high performance liquid chromatography (HLPC) was carried out using a Shimadzu (Columbia, MD) LC-20AD high performance pump interfaced with a Shimadzu SCL-20A controller. Samples were injected onto a 1.0 mm × 150 mm Ascentis-
Si silica column, with a flow rate of 70 µl/min which was eluted with a linear gradient of 100%
A (chloroform/methanol/30% ammonium hydroxide 80:19.5:0.5, by vol) to 100% B
(chloroform/methanol/water/30% ammonium hydroxide 60:34:5.5:0.5, by vol) in 14 min, then
at 100% B for 10 min (4). For quantitation multiple reaction monitoring (MRM) calibration
curves were made for each of the 6 commercially available PC-OxPL standards and peaks were normalized based on their relative responses. 10 ng of internal standard was added to all
samples during extraction. Scans were performed with transitions based on standards PONPC,
POVPC, PGPGC, KOdiAPC, and KDdiAPC with the internal standard DNPC.
Mass spectral analyses of PC-OxPL, PC, LPC and SM were performed using an AB Sciex
(Foster City, CA) 4000 QTrap hybrid quadrupole linear ion trap mass spectrometer equipped with a Turbo V ion source. Protonated adducts of the PC-OxPL were formed using the following settings: CUR, 10 psi; GS1, 40 p.s.i.; GS2, 0 p.s.i.; IS, 5500V; CAD, high; temperature, 500°C; ihe,
ON; DP, 70V; CE, 35V; EP, 15V; and CXP, 15V. Negative ionization was used for PE, PI, CL, PG and PS utilizing the following settings: CUR, 10 psi; GS1, 26 p.s.i.; GS2, 30 p.s.i.; IS, -4500V; CAD, high; temperature, 500°C; ihe, ON; DP, -80V; CE, -60V; EP, -10V; and CXP, -20V . All MRM transitions used for all phospholipids are given in Supplemental data Tables 1-2.
HPLC-MS/MS of OxCE
Four SVG samples with high plaque recovery were subjected to normal phase (NP) LC-
MS/MS, as previously described (5,6). Briefly, samples were loaded onto a 150 × 2.1 mm, 5
m,
Acentis Si column (Supelco, Bellefonte, PA) and eluted using a gradient of methyl-tert-butyl ether (MTBE) in iso-octane. Ammonium adducts were formed in the electrospray ion source by the addition of 10 mM ammonium acetate (NH
4
OAc) in 95:5 acetonitrile:H
2
O (v/v). Data were collected in MRM mode using an AB Sciex API 3200 triple quadrupole mass spectrometer (AB
Sciex, Toronto, Canada). The extent of cholesteryl ester oxidation was determined by NP-LC-
MS/MS using multiple reaction monitoring (MRM) essentially as previously published (5).
CE cations were formed through molecular ammonium ion attachment (CE+NH
4
) + .
Fragmentation of the CE cations yielded the same fragment ion regardless of the CE moiety, the cholesterol portion of the molecule minus its OH group located on carbon number 3 (C
3
-OH) yielding m/z 369.3 (C
27
H
45
) + as the major product ion. An instrument method was created to detect specified MRM pairs (e.g. m/z 690.6/369.3 for cholesteryl arachidonate, m/z
666.6/369.3 for cholesteryl linoleate, m/z 722.6/369.3 for HpETE-CEs, etc.). The first member of the MRM pair was CE[M+NH
4
+ ] and the second member was the cholesteryl cation. By coupling a liquid chromatographic separation to the mass spectrometer and employing the MRM mode, the mass spectrometer is utilized as a highly selective and highly sensitive HPLC detector.
Briefly, after calibration curves were made for each of the three major CE species and four commercially available OxCEs, aliquots of neutral lipids extracted from the recovered tissue were analyzed in the same manner as previously described (21).
The peak areas for CEs and OxCEs were normalized based on their relative response in the mass spectrometer, as determined by the calibration curves. For these analyses, only hydroxy and hydroperoxy OxCE species (as well as isobaric isomers) were considered when assessing the extent of CE oxidation.
Chemiluminescent ELISA
Extracted material sonicated and suspended in cold PBS in presence of EDTA/BHT using a
Misonix XL-2000 Sonicator (Qsonica LLC, Newtown, CT) and a 1:50 dilution was then incubated in microtiter well plates overnight at 4° C. The presence of apoB, apo(a), OxPL, and plasminogen respectively was detected by chemiluminescent ELISA with specific antibodies using
established techniques recently described (2,7,8).
Immunohistochemistry
Filters were chosen that had visible (yellow/white) material at bottom of filter. The filter bottom was cut off, the filter inverted and the material was paraffin embedded en bloc. Seven
µm serial sections were prepared, rehydrated, and immunostained for OxPL epitopes with
antibody E06 (9) and for MDA epitopes with guinea pig polyclonal antibody MDA3 (10).
Appropriate secondary antibodies were used and tissues were treated with 5 mmol/L levamisole (Sigma) and counterstained with methyl green. Controls consisted of parallel sections stained without the primary antibody and were devoid of specific staining.
Statistics
Data are presented as mean ± SD. Comparison between groups was performed with unpaired t-test.
References
1.
2.
3.
4.
5.
Bergmark C, Dewan A, Orsoni A, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res
2008;49:2230-9.
Leibundgut G, Arai K, Orsoni A, et al. Oxidized phospholipids are present on plasminogen, affect fibrinolysis, and increase following acute myocardial infarction. J Am
Coll Cardiol 2012;59:1426-1437.
Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J Biol Chem
2002;277:7010-20.
Ravandi A, Babaei S, Leung R, et al. Phospholipids and oxophospholipids in atherosclerotic plaques at different stages of plaque development. Lipids 2004;39:97-
109.
Hutchins PM, Moore EE, Murphy RC. Electrospray MS/MS reveals extensive and nonspecific oxidation of cholesterol esters in human peripheral vascular lesions. J Lipid
Res 2011;52:2070-83.
6.
7.
8.
Hutchins PM, Murphy RC. Cholesteryl ester acyl oxidation and remodeling in murine macrophages: formation of oxidized phosphatidylcholine. J Lipid Res 2012;53:1588-97.
Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apolipoprotein B-100
(OxPL/apoB) containing lipoproteins: A biomarker predicting cardiovascular disease and cardiovascular events. Biomarkers Med 2011;5:673-694.
Arai K, Orsoni A, Mallat Z, et al. Acute impact of apheresis on oxidized phospholipids in patients with familial hypercholesterolemia. J Lipid Res 2012;53:1670-8. van Dijk RA, Kolodgie F, Ravandi A, et al. Differential expression of oxidation-specific 9. epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res 2012;53:2773-90.
10. Briley-Saebo KC, Cho YS, Shaw PX, et al. Targeted iron oxide particles for in vivo magnetic resonance detection of atherosclerotic lesions with antibodies directed to oxidation-specific epitopes. J Am Coll Cardiol 2011;57:337-47.
Supplementary Table 1
All MRM transitions used in the positive ESI
774.6
786.6
772.6
784.6
782.6
766.6
780.6
814.6
826.6
812.6
810.6
818.6
808.6
842.6
840.6
838.6
836.6
834.6
832.6 m/z
PC
678.6
706.6
704.6
734.6
732.6
730.6
762.6
760.6
746.6
758.6
744.6
756.6
790.6
788.6
MRM
Transition Compound
184.3 PC (28:0)
184.3 PC(30:0)
184.3 PC(30:1)
184.3 PC(32:0)
184.3 PC(32:1)
184.3 PC(32:2)
184.3 PC(34:0)
184.3 PC(34:1)
184.3 PC(34:1 p)
184.3 PC(34:2)
184.3 PC(34:2p)
184.3 PC(34:3)
184.3 PC(36:0)
184.3 PC(36:1)
PC(36:1p)/PC(36:0
184.3 Alkenyl)
184.3 PC(36:2)
184.3 PC(36:2p)/PC(36:1Alkenyl)
184.3 PC(36:3)
184.3 PC(36:4)
184.3 PC(36:4p)/PC(36:3Alkenyl)
184.3 PC(36:5)
184.3 PC(38:2)
184.3 PC(38:3p)/PC(38:2Alkenyl)
184.3 PC(38:4)
184.3 PC(38:5)
184.3 PC(38:5p)/PC(38:4Alkenyl)
184.3 PC(38:6)
184.3 PC(40:2)
184.3 PC(40:4)
184.3 PC(40:5)
184.3 PC(40:6)
184.3 PC(40:7)
184.3 PC(40:8)
731.6
733.6
745.6
755.6
759.6
761.6
771.6
773.6
783.6
785.6
691.6
701.6
705.6
703.6
717.6
719.6
727.6
729.6
LPC
469.5
496.5
482.5
480.5
494.5
524.5
510.5
522.5
520.5
546.3
544.5
570.5
568.5
SM
663.5
673.5
675.5
677.6
687.5
689.6
184.3 LPC (14:0)
184.3 LPC(16:0)
184.3 LPC(16:0p)
184.3 LPC(16:0 Alkenyl
184.3 LPC(16:1)
184.3 LPC(18:0)
184.3 LPC(18:0e)1
184.3 LPC(18:1)
184.3 LPC(18:2)
184.3 LPC(20:3)
184.3 LPC(20:4)
184.3 LPC(22:5)
184.3 LPC(22:6)
184.3 SM C31:0
184.3 SM C32:2
184.3 SM C32:1
184.3 SM C32:2
184.3 SM C31:2
184.3 SM C31:1
184.3 SM C33:0
184.3 SM C34:2
184.3 SM C34:0
184.3 SM C34:1
184.3 SM C35:1
184.3 SM C35:0
184.3 SM C36:3
184.3 SM C36:2
184.3 SM C36:1
184.3 SM C36:0
184.3 SM C37:1
184.3 SM C38:3
184.3 SM C38:1
184.3 SM C38:0
184.3 SM C39:2
184.3 SM C39:1
184.3 SM C40:3
184.3 SM C40:2
813.7
815.7
825.7
827.7
829.7
839.7
841.7
843.7
845.7
787.7
789.7
797.6
799.7
801.7
809.6
811.7
184.3 SM C40:1
184.3 SM C40:0
184.3 SM C41:3
184.3 SM C41:2
184.3 SM C41:1
184.3 SM C42:4
184.3 SM C42:3
184.3 SM C42:2
184.3 SM C42:1
184.3 SM C43:3
184.3 SM C43:2
184.3 SM C43:1
184.3 SM C44:3
184.3 SM C44:2
184.3 SM C44:1
184.3 SM C44:0
Supplementary Table 2
All transitions used in negative ESI m/z
CL
1240.6
1446.6
1448.6
1450.6
1452.6
1472.6
1470.6
MRM transition Compound
591.6 CL 14:0 std
695.6 CL (C18:2)3 (C18:3) C72:9
695.6 CL (C18:2)4 C72:8
695.6 CL (C18:2)3 (C18:1) 72:7
695.6 CL (C18:2)2 (C18:1)2 72:6
695.6 CL (18:2)3 (20:4)1 74:10
695.6 CL (18:2)2 (18:1)1 (20:4)1 74:9
PI
865.5
863.5
861.5
859.5
857.5
855.5
853.5
885.5
883.5
793.5
781.5
809.5
807.5
837.5
835.5
833.5
881.5
913.5
911.5
909.5
907.5
PS
734.5
732.5
762.5
760.5
241.1 PI 31:1 Std
241.1 PI 30:0
241.1 PI 32:0
241.1 PI 32:1
241.1 PI 34:0
241.1 PI 34:1
241.1 PI 34:2
241.1 PI 36:0
241.1 PI 36:1
241.1 PI 36:2
241.1 PI 36:3
241.1 PI 36:4
241.1 PI 36:5
241.1 PI 36:6
241.1 PI 38:4
241.1 PI 38:5
241.1 PI 38:6
241.1 PI 40:3
241.1 PI 40:5
241.1 PI 40:6
241.1 PI 40:7
647.5 PS(32:0 std)
645.5 PS(32:1)
675.5 PS(34:0)
673.5 PS(34:1)
PG
814.6
812.6
810.5
808.5
806.5
840.6
838.6
836.6
834.5
832.5
758.5
790.6
788.6
786.5
784.5
782.5
816.6
665.5
747.5
745.5
775.6
773.5
771.5
769.5
767.5
797.5
795.5
793.5
825.6
823.6
821.5
819.5
817.5
815.5
PE
718.5
690.5
688.5
716.5
704.5
671.5 PS(34:2)
703.6 PS(36:0)
701.6 PS(36:1)
699.5 PS(36:2)
697.5 PS(36:3)
695.5 PS(36:4)
729.6 PS(38:1)
727.6 PS(38:2)
725.6 PS(38:3)
723.5 PS(38:4)
721.5 PS(38:5)
719.5 PS(38:6)
753.6 PS(40:3)
751.6 PS(40:4)
749.6 PS(40:5)
747.5 PS(40:6)
745.5 PS(40:7)
170.9 PG(14:0 std)
170.9 PG(34:1)
170.9 PG(34:2)
170.9 PG(36:1)
170.9 PG(36:2)
170.9 PG(36:3)
170.9 PG(36:4)
170.9 PG(36:5)
170.9 PG(38:4)
170.9 PG(38:5)
170.9 PG(38:6)
170.9 PG(40:4)
170.9 PG(40:5)
170.9 PG(40:6)
170.9 PG(40:7)
170.9 PG(40:8)
170.9 PG(40:9)
139.8 PE(34:0 std)
139.8 PE(32:0)
139.8 PE(32:1)
139.8 PE(34:1)
139.8 PE(34:1p)
792.5
778.5
790.5
776.5
788.5
774.5
828.5
826.5
814.5
766.5
764.5
750.5
762.5
748.5
800.5
794.5
824.5
812.5
822.5
726.5
738.5
724.5
736.5
722.5
772.5
770.5
768.5
714.5
702.5
746.5
744.5
742.5
728.5
740.5
LPE
452.3
480.3
478.3
476.3
500.3
139.8 PE(34:2)
139.8 PE(34:2p)
139.8 PE(36:0)
139.8 PE(36:1)
139.8 PE(36:2)
139.8 PE(36:2e)(36:1p)
139.8 PE(36:3)
139.8 PE(36:3e)(36:2p)
139.8 PE(36:4)
139.8 PE(36:4e)(36:3p)
139.8 PE(36:5)
139.8 PE(36:5e)(36:4p)
139.8 PE(38:1)
139.8 PE(38:2)
139.8 PE(38:3)
139.8 PE(38:4)
139.8 PE(38:5)
139.8 PE(38:5e)(38:4p)
139.8 PE(38:6)
139.8 PE(38:6e)(38:5p)
139.8 PE(40:1)
139.8 PE(40:4)
139.8 PE(40:5)
139.8 PE(40:5e)
139.8 PE(40:6)
139.8 PE(40:6e)
139.8 PE(40:7)
139.8 PE(40:7e)
139.8 PE(42:1)
139.8 PE(42:5)
139.8 PE(42:5p)
139.8 PE(42:6)
139.8 PE(42:6p)
139.8 PE(42:7)
139.8 LPE(16:0)
139.8 LPE(18:0)
139.8 LPE(18:1)
139.8 LPE(18:2)
139.8 LPE(20:4)
534.3
524.3
139.8 LPE(22:1)
139.8 LPE(22:6)
Sources of Funding
This study was funded by a grant from the Swiss National Science Foundation (to Dr.
Leibundgut), the Fondation Leducq and the National Institutes of Health R01-HL119828, R01-
HL093767, P01-HL055798, P01-HL088093, R01-HL086559, R01-HL081862 and U54-HL119893
(to Drs. Witztum, Miller, and Tsimikas) and U54-GM069338 (Drs. Dennis, Murphy and Witztum).
Disclosures
Drs. Tsimikas and Witztum are named as inventors on patents and patent applications for the potential commercial use of antibodies to oxidized LDL held by the University California,
San Diego and receive royalties from these positions. Dr. Tsimikas has received investigatorinitiated grants from Pfizer and is a consultant to Isis, Genzyme and Sanofi. Dr. Witztum is a consultant to Isis and Regulus. Dr. Miller has received an investigator-initiated grant from
Merck. The other authors report no conflicts.