Point of Care Testing - Fecal Occult Blood (FOB)
advertisement

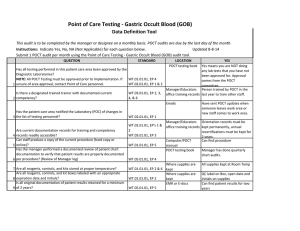
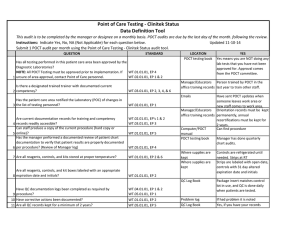
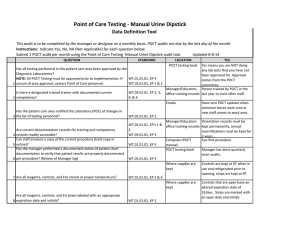
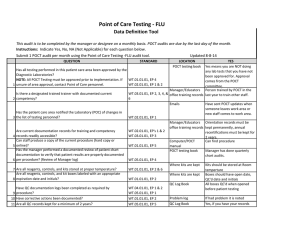
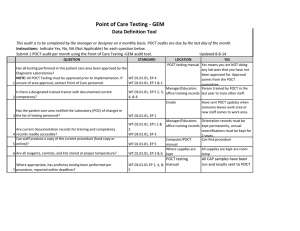
Point of Care Testing - Fecal Occult Blood (FOB) Data Definition Tool This audit is to be completed by the manager or designee on a monthly basis. POCT audits are due by the last day of the month. Instructions: Indicate Yes, No, NA (Not Applicable) for each question below. Submit 1 POCT audit per month using the Point of Care Testing -Fecal Occult Blood (FOB) audit tool. QUESTION STANDARD Has all testing performed in this patient care area been approved by the Diagnostic Laboratories? NOTE: All POCT Testing must be approved prior to implementation. If WT.01.01.01, EP 4 WT.02.01.01, EP 1 & 2 1 unsure of area approval, contact Point of Care personnel. Is there a designated trained trainer with documented current 2 competency? WT.03.01.01, EP 2, 3, 4, & 6 Updated 8-8-14 LOCATION POCT testing book YES Yes means you are NOT doing any lab tests that you have not been approved for. Approval comes from the POCT committee. Manager/Educators Person trained by POCT in the office training records last year to train other staff. Emails Has the patient care area notified the Laboratory (POC) of changes in 3 the list of testing personnel? WT.02.01.01, EP 1 Are current documentation records for training and competency 4 records readily accessible? Can staff produce a copy of the current procedure (hard copy or 5 online)? Has the manager performed a documented review of patient chart documentation to verify that patient results are properly documented 6 per procedure? (Review of Manager log) WT.02.01.01, EP's 1 & 2 WT.03.01.01, EP 3 7 Are all reagents, controls, and kits stored at proper temperature? Are all reagents, controls, and kit boxes labeled with an appropriate 8 expiration date and initials? Is all original documentation of patient results retained for a minimum 9 of 2 years? WT.01.01.01, EP 2 & 6 WT.01.01.01, EP 5 Have sent POCT updates when someone leaves work area or new staff comes to work area. Manager/Educators Orientation records must be office training records kept permanently, annual recertifications must be kept for 2 years. Computer/POCT Can find procedure manual POCT testing book Manager has done quarterly chart audits. WT.05.01.01, EP 4 WT.01.01.01, EP 2 WT.05.01.01, EP 5 Where supplies are kept Where supplies are kept EMR/Patient log/Edocs All supplies are kept at room temp Box and developer is labeled with open date and initials Patient results can be found for last two years