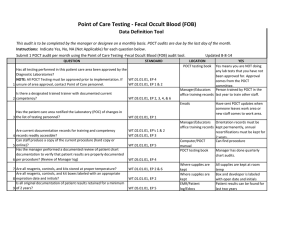
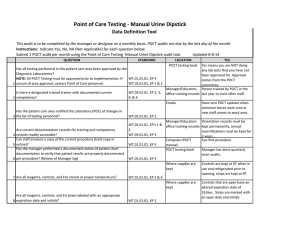
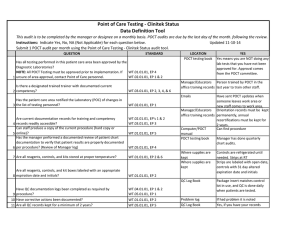
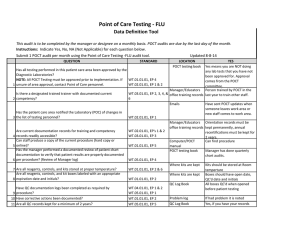
Point of Care Testing - Clostridium difficile
advertisement

Point of Care Testing – Clostridium difficle Amita Patel Guy’s and St Thomas’ NHS Foundation Trust Project Aim “Measuring the Clinical Value and impact of C. difficle Point of Care testing (POCT) in ICU and Care of Elderly wards” (Project duration 18 months) Project Stakeholders Cepheid GeneXpert System FDA approval for C. difficile 2009 First truly molecular POCT for CDI Key End Points ► Carriage and likelihood of Infection ► Predictability ► POCT of virulence – as a platform does it work? ► Turnaround times and its impact on Patient Key End points – Infection ► Measure the prevalence of carriage of (toxigenic and non-toxigenic C. difficile) ► Measure likelihood of developing infection in those that are carriers and, ► Establish if source of infection is endogenous or exogenous End points – Virulence Perspective ► Reliability of presumptive 027 identification by comparison to PCR ribotyping of tcdC deletions (and association with severity) ► Prevalence End points – POCT Perspective ► Acceptability and ease of use of platform – assessed by questionnaire to end-users ► Turnaround time of test – assumed to be time of PCR test duration ►collected by research nurse compared with a matched set of non study subjects (positive and negative) from time of test being ordered to time of result being released. End points – Disease Perspective ► Severity of CDI. Age and location matched cases ► Complications PMC, Colectomy etc ► All cause mortality / mortality related to CDI ► Length of Stay Test time Hands on time: Time to final result: approx 2 minutes approx 45 minutes Target Organisms Are Isolated, Concentrated and Washed Organism is Lysed to release DNA Disposable, enclosed Micro-fluidic Cartridge Sample is Pre filtered to remove large inhibitory debris Raw Sample and Buffers are Loaded into Cartridge (swab from stool sample) DNA Molecules Mixed with Amplification and Detection Chemicals (primers and probes) Mixture Delivered to Integrated Reaction Tube for Amplification and Detection With I-CORE Module Multiplex real-time PCR 3 Targets (plus internal positive control): ► Toxin B gene ► Binary toxin ► tcdC deletion Specimen workflow Patient Admission consent & enrolled into study Admission stool sample collected (<72 hours) Symptoms of CDI – POCT PCR GeneXpert & Positive samples sent to lab for further work Result interfaced with EPR via WinPath. Communicated to clinical team and Infection Control – appropriate management Positive Result Story so far………………………….. IT Issues: Interface Data Ownership Network Points Staffing Issues: Restricted Recruitment Bureaucracy Acknowledgement: ► ► ► Dr Simon Goldenberg Cepheid GSTS Pathology Consultant Microbiologist GSTT Foundation Trust Hospital