trypanosomoses_2_epidemoiology
advertisement

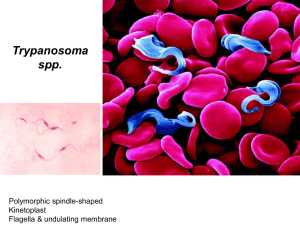
Trypanosomoses Author: Vincent Delespaux Adapted from: R.J. CONNOR and P. VAN DEN BOSSCHE, 2004, African animal trypanosomoses, in Infectious diseases of livestock, edited by J.A.W. Coetzer & R.C. Tustin. Oxford University Press, Cape Town, 12: 251 – 295 Licensed under a Creative Commons Attribution license. EPIDEMIOLOGY Trypanosomes are protozoan parasites of the genus Trypanosoma, order Kinetoplastida, and have, as characteristic organelles, a kinetoplast and a flagellum. Typically, trypanosomes are digenetic parasites and thus require two hosts to complete their life cycle: they multiply in the blood, tissues or body fluids of a vertebrate host and, with the exception of T.equiperdum which is venereally transmitted, are ingested by a haematophagous invertebrate vector. With a few notable exceptions, a cycle of development and maturation occurs in the vector, after which the parasites are transmitted to another vertebrate host as the vector feeds. Transmission is either by inoculation of trypanosomes with saliva or by contamination of mucosa or broken skin with trypanosomes in the vector's faecal material, voided during the blood meal. The type of development cycle within the vector determines whether or not infective, metacyclic parasites are present in saliva or faeces. On this basis mammalian trypanosomes are classified into the two broad sections of ‘salivaria’ and ‘stercoraria’. In Africa, the pathogenic trypanosomes that cause sleeping sickness in humans and nagana in domestic animals are salivarian, and cyclical development occurs in tsetse flies. Transmission of any trypanosome species can take place mechanically without cyclical changes occurring in the vector. In nature, this is effected by biting flies, such as Tabanus, Stomoxys and Lyperosia spp., which feed on more than one animal before repletion. Surra is a disease that affects a wide range of host animals, and it occurs in North Africa, the Near and Far East, Central and South America, Philippines and Mauritius. It is caused by Trypanosoma evansi, a dyskinetoplastic form of which — known as Trypanosoma equinum — also causes disease in equids in Central and South America where it is known as ‘mal de Caderas’ or ‘Murrina’. These parasites have adapted to an entirely mechanical, non-cyclical mode of transmission by blood-sucking flies other than tsetse. Trypanosoma theileri is a stercorarian parasite of cattle which deserves greater mention. It was first reported by Theiler in South Africa in 1903, and has since been found to occur in cattle throughout the world. It is transmitted by tabanid flies and is widely regarded as being non-pathogenic, but in certain circumstances it has been associated with disease. 1|Page Human sleeping sickness is caused by T. brucei gambiense and T. b. rhodesiense. Whilst these two subspecies do infect some domestic and wild animals, there are other, more significant pathogens of livestock. The remarkable alternate adaptations of these extracellular parasites to mammalian and insect hosts are reflected in morphological changes which are readily detectable by light microscopy. Bloodstream forms are trypomastigotes; from the posterior portion of an elongated body, some 8 – 35 µm long, arises a flagellum which extends anteriorly, and which is connected to the body by an undulating membrane. Beyond the anterior extremity of some species, the flagellum may extend free of attachment to the undulating membrane. The beating of the flagellum pulls the trypanosome forwards, imparting characteristic motility. Within the cell, in a posterior position and at the base of the flagellum, a kinetoplast is found, and a single nucleus is located almost halfway along the body. In the tsetse fly, trypomastigotes transform to epimastigotes in which the kinetoplast has migrated anteriorly, to a position adjacent to the nucleus. Differences in the morphology of the trypomastigote stages of the various species form the basis for differential diagnosis. The major characteristics are clearly seen in thin blood smears, stained with Giemsa's, Leishman's or other Romanovsky stains. a b c d Trypanosomes in thin blood smears, x1 000 stained with Diff-Quick. a = Trypanosoma congolense: note absence of free flagellum; b = Trypanosoma vivax : note long free flagellum and large kinetoplast; c = Trypanosoma brucei : note polymorphism, prominent undulating membrane and free flagellum; d = Trypanosoma brucei dividing by longitudinal binary fission. (Unpublished photomicrographs by courtesy of Dr L. Logan-Henfrey, International Laboratory for Research on Animal Diseases, PO Box 30709, Nairobi, Kenya) 2|Page Electron microphotograph of Trypanosoma congolense: cross-section showing flagellum (F), nucleus (N), mitochondrion (M) and variable surface glycoprotein coat (VSG), x86 000. Bar represents 0,2 μm. (Unpublished electron micrograph by courtesy of Dr P. Webster, Yale University School of Medicine, Department of Cell Biology, New Haven, CT) 3|Page Electron micrograph of Trypanosoma brucei : section through the flagellar pocket (FP) region of the cell. Microtubules are longitudinally sectioned, x44 000. Bar represents 0,4 μm. (Unpublished electron micrograph by courtesy of Dr P. Webster, Yale University School of Medicine, Department of Cell Biology, New Haven, CT) 4|Page Scanning electron micrograph of an intermediate (bloodstream) form of Trypanosoma brucei from the blood of a mouse. Note the prominent undulating membrane, pointed posterior end and long, free flagellum. A ‘streamer’ or filopodium can also be seen. (By courtesy of Dr P. Gardiner and reprinted by kind permission of Vinand Nantulya and Parasitology Today ) Trypanosomes show remarkable adaptation. They survive not only in the turbulent blood stream, where they face vigorous immunological assault, but they also withstand the digestive enzymes of the tsetse fly's alimentary tract. Trypanosomes reproduce by longitudinal binary fission, both in the bloodstream and in the fly, although a sexual process can apparently occur in the tsetse fly. Multiplication in each host culminates in the presence of mature trypanosomes, which stop dividing and are pre-adapted to the conditions that they will encounter in the next cyclical host. As a tsetse fly takes its blood meal from an infected host it ingests trypanosomes. Pre-adapted parasites survive in the fly, but trypanosomes that are not metabolically adapted to the new physiological conditions die. The transformation of bloodstream trypanosomes into procyclic or midgut forms within the fly’s midgut is a crucial first step in the establishment of a trypanosomal infection. The mechanism of maturation of a midgut infection is complex and, once established does not always progress to maturation. Before the infection is mature, procyclic forms transform into epimastigote and then to metacyclic forms. From the midgut, trypanosomes migrate to the mouthparts or salivary glands. 5|Page As the infective tsetse fly feeds, metacyclic trypanosomes and saliva pass through the hypopharynx and are inoculated intradermally; it is here that infection is established. From the skin, the trypanosomes reach the blood via the draining lymphatics within a few days. Trypanosomes multiply in the bloodstream, and although initially their low numbers make detection difficult, the generation time of only a few hours soon leads to high levels of parasitaemia. Trypanosomes may leave the bloodstream to reach various extravascular sites. The ability of trypanosomes to establish prolonged infections is attributable to the phenomenon of antigenic variation. Each bloodstream trypanosome is completely clad in a dense surface glycoprotein coat. Within a population of trypanosomes originating from a single infection, almost all bear the same glycoprotein coat and are thus of the same antigen type. As parasitaemia rises, a swift antibody response is elicited against the antigen type exposed on the surface of the bloodstream trypanosomes. These specific antibodies attach to the surface glycoprotein and produce complement-mediated lysis of all trypanosomes of that antigen type. However, before antibodies reach trypanolytic levels, some trypanosomes — as few as one in 100 000 — switch off the gene that controls the production of the initial surface glycoprotein and activate a gene that codes for a different protein. Trypanosomes which bear the new surface glycoprotein are of a different antigen type and are not destroyed by antibody against the first antigen type; they survive to produce another parasitaemic wave, which in turn is removed by antibody specific for that antigen type. By this time a third variant has arisen, and, escaping the effect of host antibody, it survives to produce the next parasitaemic peak. This antigenic variation is the result of sequential expression of variable surface glycoproteins (VSGs) which constitute a repertoire of variable antigen types (VATs). Infections arising from a single trypanosome may have a repertoire of more than 100 VATs. Thus shielded from total destruction, trypanosome infections usually run prolonged courses, since each VAT is present for several days before being removed. Although within a parasitaemic peak there is a mixture of a small number of VATs, the sequence of expression of VATs tends to be quite stable in clonally-derived trypanosomes. This imparts immunologically distinct characteristics to a strain of trypanosomes, the distinct strain being called a ‘serodeme’. In the course of successive parasitaemic waves, some trypanosomes stop dividing and transform to the pre-adapted form able to survive in the tsetse. After ingestion by the tsetse, pre-adapted trypanosomes shed the glycoprotein coat, transform, multiply and finally mature. Infective tsetse then transmits metacyclic trypanosomes to another host. Irrespective of the VAT of the bloodstream trypanosomes ingested by a fly, the metacyclic VATs of a serodeme are relatively constant. The antigenic diversity within a species leads to the possibility of animals in a tsetseinfested area being exposed to a large number of antigenically distinct trypanosomes, but although trypanosomes within a species may be antigenically dissimilar, they are morphologically indistinguishable. The characterization of trypanosomes relied for a long time on comparisons of their morphology, motility, host specificity, tsetse transmissibility and their development within the fly, but more recent characterization methods include isoenzyme typing, analysis of kinetoplast DNA by polyacrilamide gel electrophoresis, pulsed field gradient electrophoresis of chromosomal digests and DNA hybridization. 6|Page The sequel to infection with salivarian trypanosomes is not always disease. The outcome is determined by many factors, frequently related to the susceptibility of the host and the pathogenicity of the trypanosome. In the case of wild animals, a natural cycle of trypanosome transmission occurs which is not associated with disease. Similarly, in some breeds of domestic animals, infection with salivarian trypanosomes is tolerated, and host and parasite reach an equilibrium. Disturbance of the equilibrium may precipitate disease a long time after establishment of the infection. Thus, although the tsetsetransmitted trypanosomes are aetiological agents of African trypanosomosis, infection is not always synonymous with disease. The occurrence of T. theileri in healthy cattle throughout the world exemplifies a well-developed host-parasite relationship. Similar events occur in many wild animals which harbour tsetse-transmitted trypanosomes. Infected animals show no clinical signs, but when they are subjected to the stress of capture, for example, their immunity is reduced, parasitaemia flares up and clinical disease may be precipitated. The epidemiology of African trypanosomosis is almost entirely dependent on tsetse flies. African trypanosomes are well-adapted parasites of many species of wild animals, and sylvatic cycles of trypanosome transmission occur throughout the 10 million square kilometres infested by this unique vector. The natural hosts of salivarian trypanosomes usually show no clinical signs of infection, host and parasites being in equilibrium. The large numbers of naturally infected wild animal hosts constitute a huge reservoir of trypanosomes. Once infected, tsetses remain so for life and thus they too form a reservoir of infection. Consequently, when domestic animals are introduced into areas in which sylvatic cycles of trypanosome transmission occur, trypanosomosis always emerges as a serious disease. Wild animals are the natural hosts of T. brucei rhodesiense, the aetiological agent of human sleeping sickness in central, eastern and southern Africa. Thus, people living and working in tsetse areas are at risk of contracting the disease, but for animal trypanosomosis to occur it is not always necessary for livestock to enter tsetseinfested areas; tsetse also move. Changes in land use may also alter the extent of tsetse infestation. The abandonment of cultivation, for various reasons, permits the regrowth of vegetation, which may then provide suitable tsetse habitat. Conversely, the intensive settlement and cultivation seen in some areas destroy tsetse habitat. The trypanosomal infection rate in tsetse is of prime importance. The ease with which infections develop in tsetse depends upon the fly’s vectorial capacity and specific factors related to the blood of host animals. Generally speaking, the duration of the development of trypanosomes in tsetse increases with increasing complexity of the developmental cycle. The prevalence of trypanosomal infections in tsetse is also affected by host preference. Two aspects are important in this context: first, there is diversity of host preference among Glossina and, second, different species of hosts vary in their susceptibility to infection with the different species of trypanosomes. Of particular importance is the relationship between absolute tsetse density and biting rate. A good understanding of this relationship is essential when predicting the impact of tsetse control interventions. Usually the challenge increases with the tsetse population density but even at low densities tsetse can 7|Page still cause a substantial disease problem. This is partly attributed to the often observed, increased frequency with which flies that have metacyclic infections in their mouthparts probe. Management practices may also alter the challenge to which livestock are subjected, and in this sense management is central to the epidemiology of trypanosomosis. The different management of calves and adult cattle can reduce significantly the level of challenge to which young animals are subjected. Observations on the grazing ranges of livestock in West Africa showed that while cattle foraged widely in tsetse-infested habitat, sheep, goats and donkeys remained closer to the villages. As a result, small ruminants and equids were less exposed to attack by tsetse than cattle. A major influence on the epidemiology of trypanosomosis is the use of trypanocidal drugs. Whilst permitting the use of tsetse-infested land, chemotherapy may alter the prevalence of trypanosome species in the area. In Zimbabwe, it was reported that the use of diminazene increased the prevalence of T. vivax in cattle. Furthermore, the repeated use of trypanocides may result in the emergence of drug resistant strains of trypanosomes, and the epidemiological picture then changes. Wild animal hosts of tsetse, such as kudu, warthog, bushbuck, bushpig, buffalo, elephant and rhinoceros, are vital to sylvatic cycles of trypanosome transmission and they form a major reservoir of infection. The increasing popularity of game ranching or farming frequently entails the translocation of these animals from tsetse habitats to tsetse-free areas. As a result, clinical disease may occur in animals normally regarded as ‘immune’, in the absence of the tsetse vector. There is also the possibility that trypanosomes could be mechanically transmitted from these species to livestock. Wild animal hosts of tsetse and certain West African taurine cattle are tolerant of tsetse-transmitted trypanosomes. Carefully controlled experiments have shown that the trypanotolerance of N’Dama cattle and of African buffalo (Syncerus caffer) is an innate characteristic. This trait also occurs in some breeds of sheep and goats. Lower trypanosomal parasitaemias and less severe anaemia occur in trypanotolerant livestock than in trypanosusceptible animals. The epidemiological significance of this trait lies in the lower morbidity and mortality due to trypanosomosis in tolerant breeds. Although trypanosome-infected animals may effect self-cure, the usual sequel to infection in tolerant animals is the establishment of a balance between host and parasite. If the host is stressed, the equilibrium is disturbed and a clinical episode of variable severity is precipitated. Stress takes many forms. Animals in late pregnancy or which are lactating are more susceptible to trypanosomosis. Overwork also constitutes a stress, which in infected trypanotolerant stock may precipitate disease. This has serious consequences for animal traction and for hard-working bulls used in restricted breeding seasons in tsetse-infested areas. In a similar manner, intercurrent disease is stressful; trypanosome-infected animals with helminthosis or other diseases are more severely affected than those with either disease alone. The combined effect of poor nutrition and increased exercise is often associated with an increased incidence of trypanosomosis in the dry season. Age also has a significant effect on resistance to trypanosomosis. It is widely recognized that cattle born in an infested area do not immediately succumb to disease, even though they acquire trypanosomal infections when young, whereas cattle brought into the area readily succumb. 8|Page Despite the antigenic complexity of trypanosomes, infected animals do mount immune responses which, especially when supported by chemotherapy, can confer specific protection against homologous serodemes. Thus, within a defined area, animals may acquire protective immunity against locally prevalent serodemes. However, the movement of these animals to another area may expose them to different strains, or serodemes, to which they may succumb. This type of tolerance requires continuous challenge and explains the low mortality of cattle in high challenge, endemic areas (such as the Eastern Province of Zambia) where trypanocidal drugs are used to prevent animals from succumbing to nagana. The introduction of tsetse control measures in such areas would result in a loss of immunity that would render the population highly susceptible to infection and disease. If tsetse control measures breakdown severe outbreaks of trypanosomosis would ensue, probably with high mortality. The African continent is experiencing considerable environmental changes as a result of an unprecedented demographic growth.The increasing human pressure and the demand for arable land is a major driver of land-use change resulting in deforestation, erosion and loss of biodiversity and suitable habitats for tsetse flies Like many other arthropods, tsetse flies rely for their survival on habitats with suitable conditions.The destruction and fragmentation of habitats has had important repercussions for the density and distribution of tsetse flies. Insome instances,the drastic changes have resulted in the disappearance of certain species In many cases, however, tsetse flies persist at lower densities in what might appear to be unsuitable environments probably occupying microclimatic niches. The effects of anthropogenic environmental changes on the epidemiology and impact of livestock trypanosomosis are largely driven by the degree with which the tsetse flies adapt to an environment where domestic animals are becoming increasingly important for their survival.These changes have been demonstrated in southern Africa where epidemiological studies have clearly revealed the effect of such anthropogenic changes on the impact of the disease on livestock and the appropriatenesss of particular control strategies. 9|Page