WS #6 - What Is Molar Mass?
advertisement

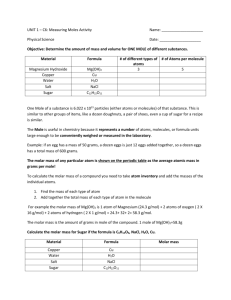
Scientist: Per: Date: UNIT 5: THE MOLE ____________________ _________ _________ #6 WHAT IS MOLAR MASS? The mole concept is fundamental to the understanding the world of atoms and molecules. It allows us to count incredibly large numbers of impossibly small particles. Further, the mole acts as a “bridge” between an exact quantity of atoms and its mass. The Mole 1.The mole (mol) is a unit for counting objects. It is especially useful for counting tiny objects like atoms, molecules, and ions. 1 mole of objects = 6.02 x 1023 objects 2. How many atoms of copper are in 1 mole of copper? 3. How many molecules of water are in 1 mole of water? 4. How many atoms of zinc are in 10 moles of zinc? 5. Find out many moles of water contain 1.5 x 1024 molecules of water. Atomic Mass (The mass of individual atoms or particles) Atoms and molecules are extremely small particles. The mass of individual atoms and molecules is usually provided in units called “atomic mass units” or amu. The conversion factor for amu and grams is provided below. 1 amu = 1.67 x 10-24 g 1. What does amu stand for? 2. How is amu defined? 3. Why is the mass of individual atoms provided in amu and not grams? Consider the masses of the following elements when answering the questions below. 1 6 Hydrogen 1.01 Carbon 12.01 H 11 Na C Sodium 22.99 4. What is the atomic mass of a hydrogen atom? Include units. ________ 5. What is the atomic mass of a carbon atom? Include units. ________ 6. What is the atomic mass of a sodium atom? Include units. ________ Molar Mass (The mass of a mole of particles) Scientists do not actually measure the mass of atoms in amu because individual atoms are far too small to see or to weigh. Furthermore, in reality there is no lab equipment sensitive enough to measure in amu… so such measurements would be impossible. Using the mole however… measuring billions of trillions of atoms at a time... the mass of atoms can be converted into grams, which is a far more convenient unit for measuring in the lab. Grouping atoms into moles allows scientists to work with meaningful amounts of substances and allows meaningful measurements in grams. Molar mass is defined as the mass of one mole of any substance. It is expressed in grams per mole (g/mol). 7. Why is amu not a useful unit of measurement for scientists in the lab? How do scientists weigh atoms in the lab? Calculating Molar Mass: Let’s start by converting the atomic mass of hydrogen (in amu) into its molar mass (in grams) 8. Find the molar mass of hydrogen. Use the following conversion factors to determine the mass of one mole of hydrogen atoms in grams. Defined relationships: 1 amu = 1.67 x 10 -24 g 1 mol H = 6 x 1023 atoms H 1 atom H = 1 amu 1 𝑚𝑜𝑙 𝐻 1 x 6 𝑥 1023 𝑎𝑡𝑜𝑚𝑠 𝐻 1 𝑚𝑜𝑙 𝐻 x 1 𝑎𝑚𝑢 𝐻 1 𝑎𝑡𝑜𝑚 𝐻 x 1.67 𝑥 10−24 𝑔𝑟𝑎𝑚𝑠 1 𝑎𝑚𝑢 = ___________________grams 9. Find the molar mass of carbon. Use the following conversion factor to determine the mass of one mole of carbon atoms in grams. Defined relationship: 1 amu = 1.67 x 10 -24 g 1 𝑚𝑜𝑙 𝐶 1 x 6 𝑥 1023 𝑎𝑡𝑜𝑚𝑠 𝐶 1 𝑚𝑜𝑙 𝐶 x 12 𝑎𝑚𝑢 𝐶 1 𝑎𝑡𝑜𝑚 𝐶 x 1.67 𝑥 10−24 𝑔𝑟𝑎𝑚𝑠 𝐶 1 𝑎𝑚𝑢 𝐶 = ___________________grams C 10. Find the molar mass of sodium. 12. Using what you have learned so far, fill the blanks below. Include units. Molar mass of Pb = ______ Molar mass of Si = ________ Molar mass of Cl = __________ Molar Mass of Molecules Molecules are groups of atoms. The molar mass of a molecule is the combined molar masses of all of the atoms in the compound. Example: 1 mole of NaOH = 1 mole of Na 23g + 1 mole of O 16g Molar mass of NaOH = 40 g/mol 13. Calculate the molar mass of water: H2O 14. What is the molar mass of sulfur dioxide: SO2 15. What is the molar mass of ammonia: NH3 16. What is the molar mass of glucose: C6H12O6 17. What is the molar mass of magnesium hydroxide: Mg(OH)2 18. What is the molar mass of ammonium phosphate: (NH4)3PO4 + 1 mole of H 1g = 40g