Tritation lab Analysis question #2: What is the molarity of a nitric acid
advertisement

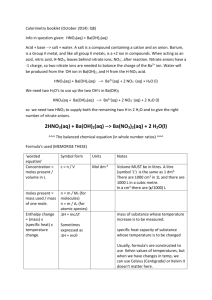
Tritation lab Analysis question #2: What is the molarity of a nitric acid, HNO3, solution if 43.33 ml of 0.1000M KOH solution is needed to neutralize 20.00 ml of the acid solution? Step 1: write the neutralization equation HNO3 + KOH KNO3 + H2O 1 mole of KOH reacts with 1 mole of HNO3 (1:1 mole ratio) Step 2: Use dimensional analysis to solve for moles of KOH: Moles of KOH = 0.1000 mol KOH X .04333L = 0.004333 moles of KOH 1L 1 Moles of HNO3 = 0.004333 moles KOH X 1 mole HNO3 = 0.004333 moles HNO3 1 1 mole KOH Step 3: Use dimensional analysis to solve for moles/L of HNO3: [HNO3] = 0.004333 moles HNO3 = 0.217 M 0.020L