AcuteToxinSOP_Template
advertisement

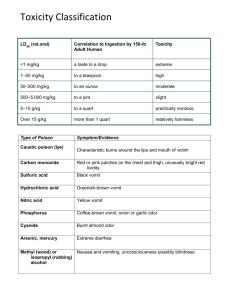
Acute Toxin Standard Operating Procedures For working with: Toxin Name: Building/Room: P.I. Name: EH&S Approval: Revision #: Effective Date: P.I. Signature: Approval Date: Note: The UF EH&S Biosafety Office oversees the proper acquisition, use, storage and disposal of unfractionated mixtures and purified preparations of biological toxins with a mammalian LD50 ≤ 100µg/kg body weight (referred to as Acute Toxins), as well as natural and recombinant organisms which produce these biological toxins. All research projects involving an acute toxin(s) must be registered with the Biosafety Office using the Acute Toxin Registration Form. This document is intended to be adapted to the lab as a site-specific document. Additional precautions or regulations may be required per risk assessment by the Biological Safety Office and/or Institutional Biosafety Committee and/or Responsible Official. This SOP must be submitted to the Biosafety Office with your Acute Toxin Registration Form and must be approved by the UF EH&S Biosafety Office BEFORE work can begin with the toxin. If you are working with multiple toxins, you must indicate site-specific and toxin-specific procedures if you choose to use a single SOP to cover all toxins used in your lab. This SOP should be revised whenever significant changes to the experimental protocol are made and/or at least annually. All revisions and annual reviews will require approval by the EH&S Biosafety Office and must be read, understood and signed off by all personnel working with the toxin. 1. Circumstances of Use: See “Instructions for Creating a Lab-Specific SOP.” 2. Potential Hazards: Routes of Exposure: Target Organs: Signs/Symptoms of Exposure: Incubation period: Toxicological data (by route of entry): Fill in the above information. See “Instructions for Creating a Lab-Specific SOP” for additional guidance. 3. Medical Considerations: See “Instructions for Creating a Lab-Specific SOP.” 4. Security Procedures: The following security measures are required for work with Acute Toxins: Only minimum amounts of toxin(s) will be kept on hand. All toxin orders should be sent in such a manner in which the package can be tracked and requires your signature for receipt (we strongly recommend using FedEx). Toxin stocks and solutions must be secured at all times when not in use. o Toxin is stored in: (fill in equipment and storage site) o The key or combination to the lock is under the control of the P.I. o If a lock box is used, the box must be affixed to the refrigerator or freezer or the refrigerator or Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 1 of 17 2/24/13 freezer must also be locked. Toxin Inventory: o A hardbound log book must be used to record the inventory. Each batch of toxin should be recorded on a separate page (see Appendix A for sample log book entry). The following information should be recorded at the top of the page: Toxin Name, Date Received, Source or Received From, Amount Received, Form Received (Liquid or Powder), Concentration of Liquid Stock, Total Volume The following headings should be used to track the Removal/Use of Toxin: Starting Amount, Amount Removed/Used, Date of Removal/Use, Purpose, Removed by (Print Name), Signature and Amount Remaining. o The inventory book should also contain a section to record the destruction/inactivation of toxin stock. The following headings should be used: Toxin Name, Date Destroyed, Amount Destroyed, Method of Destruction, Destroyed by (Print Name), Signature, Witnessed by (Print Name), Signature, Amount remaining (if any) o All entries in the book shall be done in ink. o The Inventory book must be kept in a secure location (e.g., locked drawer) and the key or combination shall remain under the control of the P.I. o Discrepancies, alterations, or suspicious entries must be reported to the P.I. and the Biological Safety Office (392-1591) immediately. o EH&S staff will inspect the inventory and log books on a periodic basis. Add additional security practices following the guidance provided in “Instructions for Creating a Lab-Specific SOP.” 5. Administrative Controls: All non-essential personnel must be excluded from the work area when toxins are in use. o Post the “Toxins in Use” sign (see Appendix B) to warn/exclude personnel not directly involved in the toxin work. All personnel working with the toxins should be familiar with the MSDS sheet for each toxin and signs and symptoms of exposure. The “buddy system” is required in the following instances so that assistance is immediately available if needed: o Work with concentrations or amounts near or exceeding the LD50 dose for a human o High risk activities with acute toxins including: Procedures that generate or potentially generate aerosols (e.g., vortexing, grinding, centrifuging, intra-nasal inoculation of animals, sonication, etc.) Utilization of concentrated stocks or large quantities of toxins Work with powdered or dried toxins Any work with highly lethal toxins or highly purified forms of less lethal toxins Use of needles or sharps in experiments with toxin o Based on any recommendation provided by the Biosafety Office, IBC and/or RO/ARO. 6. Engineering Controls: All toxins should be handled in the operationally effective zone of a certified biological safety cabinet (BSC) or fume hood for all procedures where aerosols may be produced o Aerosols may be produced during routine laboratory procedures (e.g., vortexing, mixing, pipetting) and during open or pressurized manipulations of suspensions. Add additional engineering controls using the guidance provided in “Instructions for Creating a Lab-Specific SOP.” 7. Safe Work Practices: All packages containing the toxin should be opened in a certified biosafety cabinet or fume hood. o Inspect the toxin container for damage during shipping. Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 2 of 17 2/24/13 If damaged, decontaminate and dispose of immediately following procedures established in Section 10: Waste Disposal; contact vendor. If not damaged, packaging may be discarded in the regular trash. o Log receipt of toxin shipment immediately in the Toxin Log book and secure the toxin. Experiments should be designed to eliminate or minimize work with dry toxins. o Liquid stocks should be purchased when available since toxin in powdered form is more hazardous than toxin in liquid form. Minimize the use of sharps; safety sharps should be considered (and may be required per risk assessment). At a minimum: o Luer-lock syringes are required o Glass Pasteur pipettes are prohibited. Set up the work area with the appropriate equipment prior to the start of all experiments: o Place a blue absorbent, plastic-backed pad sprayed with an appropriate disinfectant in the work area of the BSC (be careful not to block the front or rear grills). Conduct all work with toxin over the disinfectant-soaked pad.. o Ensure that containers for solid waste (pipette tips, etc) and/or liquid waste (filled with appropriate disinfectant) are accessible inside the BSC or fume hood. o Toxin-specific spill kit is readily accessible o Sharps containers are accessible within BSC or fume hood (if required) o All PPE Disposable materials should be used whenever possible for work with toxins; containers that will be re-used will require extensive decontamination prior to washing. The interior of the BSC or fume hood and all equipment (e.g., pipettors) should be thoroughly decontaminated after work with toxins with (list appropriate disinfectant and concentration). Remove and dispose of, or decontaminate, PPE and wash hands with soap and water before leaving the work area. Add additional precautions following the guidance provided in “Instructions for Creating a Lab-Specific SOP.” 8. Personal Protective Equipment (PPE): Long pants and full-coverage shoes are required for work with toxins. A laboratory coat or gown must be worn at all times when working with toxins. Cloth lab coats may not be taken home for laundering; they must first be autoclaved prior to transport to a UF-approved laundering service. Disposable lab coats are strongly recommended. Safety glasses with side shields or goggles should be worn when working with toxins. o Safety glass should be decontaminated after each use with an appropriate disinfectant prior to reuse. Two pairs of disposable latex and/or nitrile gloves should be worn when working with toxins. o Alternating nitrile gloves as the inner pair and latex gloves the outer pair will make glove changes easier. o Contaminated gloves should be removed carefully as soon as possible and placed into an appropriate disinfectant or autoclavable bag that is then closed. A new pair of outer gloves is donned. Personnel responding to spills outside of a biocontainment device are required to wear all of the above PPE and an N95 respirator. The use of a respirator requires both a medical clearance and a fit test to be worn. Contact UF EH&S at 392-1591 for more information. Add additional PPE requirements following the guidance provided in “Instructions for Creating a Lab-Specific SOP.” 9. Storage, Transportation and Shipping: When stored in the laboratory, toxin containers must be sealed, legibly labeled (toxin name and hazard warning at a minimum) and secured (see Section 4: Security Procedures) to ensure restricted access. Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 3 of 17 2/24/13 o Individual tubes of toxin dilutions should also be stored in sealed, labeled secondary containers (e.g., Eppendorf tubes of toxin dilutions can be placed in a 50ml conical tube that is appropriately labeled with toxin name, concentration and biohazard sticker). Refrigerators and/or other storage containers housing biological toxins should be labeled with contact information for trained, responsible laboratory personnel. Toxins and toxin solutions should be transported in closed, double or triple containment (e.g., small tubes within a large conical tube inside a Tupperware container, or a vial in a sealed bag) with absorbent and cushioning material. All storage and transport containers must be labeled with a biohazard sticker. Transport of toxins should occur through low-traffic hallways whenever possible. Shipping biological toxins requires current training certification in IATA/ICAO and DOT regulations. Contact the UF Biosafety Office (392-1591) for more information. Add additional precautions following the guidance provided in “Instructions for Creating a Lab-Specific SOP.” 10. Decontamination and Waste Disposal: Decontamination – the following information must be included (at least one method listed): Name and concentration of decontamination solution: fill in Time for effective decontamination: fill in Physical inactivation (method, temperature, time): fill in Disposal of Toxin-Contaminated, or Potentially Contaminated Liquid Waste For collection of liquid waste during a toxin experiment, set up a liquid waste container in the BSC. o The container should be a durable, disposable, leak-proof container. For example, for small amounts of liquid waste- a 15ml or 50ml conical tube could be used to collect liquid waste. o Add an appropriate amount of (insert decontamination solution, 2X concentration) to the container and decant or pipet liquid waste into the container during the experiment. Care should be taken to minimize aerosols and splashes. Dispense liquid waste from a pipet by holding the pipet tip against the side of the collection container or submerging the tip directly in the disinfectant solution. At the completion of the experiment, place the primary waste container into a secondary, durable, leakproof container and seal. o Fill out a yellow hazardous (chemical) waste label indicating concentration of components in the waste bottle (including the amount and concentration of bleach) and affix to the bottle. o Store the doubly-contained inactivated toxin in the designated hazardous waste accumulation area (fill in location). Do not store toxin waste for prolonged periods of time. Toxin waste should be picked up ASAP as a best management practice. To schedule a hazardous waste pick up call EH&S (392-8400) or schedule on-line. EH&S Hazardous Waste will not pickup toxin materials that have not been inactivated. Disposal of Toxin-Contaminated, or Potentially Contaminated Solid Waste See “Instructions for Creating a Lab-Specific SOP.” Decontamination/Cleaning of Toxin-Contaminated, or Potentially Contaminated Reusable Labware See “Instructions for Creating a Lab-Specific SOP.” Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 4 of 17 2/24/13 11. Inactivation and Disposal of Liquid Toxin Stock Solutions: Note: All destruction or inactivation events of concentrated toxin stock solutions require a witness and must be documented in the Inventory Log. Destruction/Inactivation of select agent toxin stock material associated with a select agent-registered project must be witnessed and documented by the UF RO/ARO. For all other destruction/inactivation events, the witness may be a second member of the laboratory approved for toxin use. Donn PPE as described in section 8: Personal Protective Equipment. Place plastic-backed absorbent pad in the working area of the BSC (be careful not to block the front or rear grill) and spray with disinfectant. Place primary toxin container in a secondary plastic disposable container with a sealable lid. Carefully add an equal volume of (insert decon solution, concentration) into the toxin solution. Allow a minimum of 1 hr contact time to inactivate the toxin. o Replace cap on primary container and secure the lid on secondary container. o Fill out a yellow hazardous (chemical) waste label indicating concentration of components in the waste bottle (including the amount and concentration of bleach) and affix to the bottle. o Store the doubly-contained inactivated toxin in the designated hazardous waste accumulation area (fill in location). Do not store toxin waste for prolonged periods of time. Toxin waste should be picked up ASAP as a best management practice. o To schedule a hazardous waste pick up call EH&S (392-8400) or schedule on-line. EH&S Hazardous Waste will not pickup toxin materials that have not been inactivated. 12. Spill Response: Spill Response: 1. Spills of acute toxins inside BSC (any volume, concentration): Note: If the spill extends into the airflow grill and drain pan of the BSC, contact the PI, BSO or EH&S before proceeding. Remove any contaminated PPE/clothing and dispose of in a biohazard bag; close bag. o If you are contaminated- immediately wash the affected area with soap and water. o Replace PPE and alert others in area to spill. Cover spill with paper towels or other absorbent material. Apply (list decon solution and concentration) to the spill, starting at the perimeter and moving towards the center of the spill area. Allow disinfectant to sit for a minimum of 30 minutes. Pick up absorbent materials and wipe up excess decontamination solution; dispose of in a biohazard bag. Use tweezers, forceps or tongs to pick up sharps and place in sharps container. Wipe walls and/or cabinets that may have been splashed with (List decon solution and concentration). Rinse all decontaminated surfaces with water followed by 70% Ethanol. Dispose of paper towels in appropriate waste container. Follow the appropriate Waste Disposal Procedures as outlined in Section 10: Decontamination and Waste Disposal. Wash hands and exposed skin with soap and water before exiting laboratory. Notify PI of the spill. If appropriate, the PI will inform the Biological Safety Officer. Report any potential exposure to the Student Health Care Center. 2. Spills of acute toxins outside the BSC (any volume, concentration): Alert all personnel in the room of the spill and immediately evacuate to the hallway. o Remove any contaminated PPE at the doorway to the hall, if possible. o Decontaminate shoes, if necessary. o If you are contaminated, immediately wash the affected area with soap and water. Secure the area and post a “Spill: Do Not Enter” sign on the laboratory door. Immediately notify the PI and the Biological Safety Officer or EH&S of the spill Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 5 of 17 2/24/13 o Do not re-enter the laboratory until you have consulted with biosafety personnel. o Prior to re-entry into the contaminated lab, Biosafety personnel will develop and implement a decontamination and clean-up plan. Report any potential exposure to the Student Health Care Center. 13. Exposure Management: Location of nearest Emergency Eyewash: fill in location Location of nearest Emergency Shower: fill in location Skin or Wound Exposures: Move to (or move person to) an uncontaminated area. Remove contaminated clothing. Wash skin and wound exposures with fresh 0.5% sodium hypochlorite or soap and water. For spills of large quantities of toxin onto clothing or skin, immediately use the emergency shower and follow with fresh 0.5% sodium hypochlorite or soap and water. o Place contaminated clothing in a red biohazard bag for decontamination. Eye or Mucous Membrane Expsosure: Use emergency eyewash to immediately flush eyes with gently flowing, potable water for a minimum of 15 minutes o Forcibly hold eye open to ensure effective rinsing behind eyelids. Move eyes side-to-side and up-anddown during rinsing o Remove contact lenses. In all instances: Obtain medical attention as required: o Working hours: Student Health Care Center (SHCC) on main campus or satellite SHCC in Dental Tower (D2-52) o After-hours: Call 911 or go to the nearest medical facility Review the MSDS for symptoms of exposure or delayed onset effects. Report Incident to Supervisor (insert supervisor and phone #) and Biological Safety Office (392-1591). Contact UF Worker’s Compensation (352-392-4940). Follow spill cleanup procedures (Section 13) for contaminated surfaces and materials. 14. Personnel Training: In addition to General Biosafety Training, all laboratory personnel working with acute toxins must be trained in the theory and practice of the toxin being used with special emphasis on the nature of the practical hazards associated with laboratory operations; this includes how to handle transfers of liquids containing the toxin, waste disposal, decontamination of materials, equipment and work surfaces after routine laboratory operations and appropriate spill response procedures. Furthermore, all personnel must read and fully adhere to this SOP when handling the toxin. Add any additional laboratory-specific training required for work with toxins. 15. Animal Use of Biotoxins Please check one of the following: ☐ We will not be dosing animals with the toxin. ☐ We will be dosing animals with the toxin. If you checked the second box and will be dosing animals with the toxin, the “Animal Use of Biotoxins” section (Appendix C) must be completed and followed. The Animal section includes requirements related to notification and coordination of Animal Care Services (ACS), room and cage labeling and health considerations for animal caretakers. Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 6 of 17 2/24/13 “I have read and understand this SOP. I agree to fully adhere to its requirements.” Last Name First Name UFID# Signature Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 7 of 17 Date 2/24/13 Instructions for Creating a Laboratory-Specific Standard Operating Procedure (SOP) for Acute Toxins Use fillable form starting on page 1 to create your lab-specific SOP. Include relevant information from all sections. Risks should be assessed for each toxin individually to determine the appropriate controls, personal protective equipment, and decontamination procedures for preparing, handling and disposing of the toxin. These instructions are meant to provide guidance in creating your laboratory-specific SOP and may not address every possible safety and/or security concern. You should use these instructions, along with your toxin risk assessment and any guidance from the Biosafety Office to ensure that you address all potential hazards and means of mitigating those hazards. 1. Circumstances of Use: Provide a brief description of the work and what the toxin will be used for. Include the following in your description: Quantity required, approximate frequency of use and location(s) of use. o For select agent toxins- ensure that the cumulative amount of toxin under the control of a PI does not exceed the permissible toxin amounts. Describe concentrations used and how needed concentration and amount will be prepared. Indicate whether the toxin will be purchased in liquid or powder form. o Liquids are generally less hazardous than powders; purchase acute toxins in liquid form if possible. o Consider purchasing dilute solutions when possible. 2. Potential Hazards: Fill in the required information for each toxin. Possible routes of entry: o Consider: skin, inhalation, mucous membranes, ingestion, auto-inoculation, etc. o For each potential route of exposure- indicate how/when exposure might occur (e.g., inhalationduring weighing, mixing or splashes; needle sticks from injection preparation and administration, etc). The following toxins have summary statements in the Toxin Agent section of the 5th edition of the NIH/CDC’s Biosafety in Microbiological and Biomedical Laboratories manual which may be helpful: o Botulinum neurotoxin o Staphylococcal enterotoxins o Ricin toxin o Selected low molecular weight toxins T-2 mycotoxin Saxitoxin and related paralytic shellfish poisons Tetrodotoxin Brevetoxin Palytoxin Polypeptide conotoxins Microcystin-LR The Toxin, Toxin-Target Database has detailed toxin information that may also be helpful in determining potential hazards. If you are having difficulty finding literature or guidance for your particular toxin, contact the EH&S Biosafety Office for assistance (392-1591). 3. Medical Considerations: List any toxoid vaccinations or antitoxins required or recommended for this toxin. Consult with EH&S Biosafety Office if you need assistance. Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 8 of 17 2/24/13 o If antitoxins or toxoid vaccinations are required, all personnel planning to work with the toxin must contact the Student Health Care Center at 352-294-5700 to discuss these considerations. If there are no specific medical considerations recommended for work with the toxin, please put “No special medical considerations recommended.” 4. Security Procedures: If you are working with any amount of a select agent toxin, you must adhere to the Toxin Due Diligence Provision for Select Agent Toxins. 42 CFR 73.3(d) requires that select agent toxins may only be transferred after the transferor uses due diligence and documents that the recipient has a legitimate need (i.e., reasonably justified by a prophylactic, protective, bona fide research, or other peaceful purpose) to handle or use the toxin. If you are working with a select agent toxin- copy and paste the following into the Section 4: Security – All PIs in possession of any quantity of a Select Agent Toxin must retain documentation of all transfers of any quantity of the toxin outside of the laboratory; documentation should be retained by the PI for a minimum of three (3) years. The required documentation must include the following: o Name of the recipient, institution, address, telephone # and email address; o Toxin and quantity transferred; o Purpose of use/knowledge of recipient’s legitimate need for the toxin. This can be obtained either by requiring the recipient to complete documentation stating their intended use of the toxin OR by the transferor documenting their knowledge of the recipients legitimate need for the toxin. Reporting Suspected Violations or Suspicious Activity. If a PI in possession of any quantity of a Select Agent Toxin detects suspicious activity associated with a request for toxin or suspicious activity associated with a shipped toxin, s/he must immediately notify UF’s Responsible Official (RO) or Alternative Responsible Official (ARO) who will then contact the Federal Select Agent Program. o Alternatively, anonymous reporting is possible through the Office of the Inspector General: Anonymous OIG Hotline: 1-800-HHS-TIPS (800-447-8477) Web: http://oig.hhs.gov/fraud/hotline When reporting issues to the OIG, ensure that you indicate this is a Select Agent Program Issue. 5. Administrative Controls: Add any additional administrative controls per your toxin risk assessment or the recommendation of the Biosafety Office. 6. Engineering Controls: Engineering controls should be selected according to the risk assessment for each specific toxin operation. List the engineering controls required for this toxin (e.g., HEPA filtered enclosure inside chemical fume hood or Class III Biological Safety Cabinet may be required for experiments that intentionally aerosolize toxin). Resources that may be useful in determining appropriate engineering controls include: Appendix I of the BMBL, 5th edition: Guidelines for Work with Toxins of Biological Origin Section VIII-G: Toxin Agents of the BMBL, 5th edition “Safety and Health Considerations for Conducting Work with Biological Toxins.” Applied Biosafety, 6(3) pp. 117-135, 2001. EH&S Biosafety Office (392-1591) Things to consider when listing Engineering controls for toxins (include all that apply): Intentional generation of aerosols is considered a high-risk operation that requires approval by the Biosafety Office (392-1591) to ensure that appropriate engineering controls are in place. Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 9 of 17 2/24/13 Low molecular weight (LMW) toxins or work involving radionucleotides combined with toxins may require the use of a charcoal-filter in addition to HEPA filtration. Work with toxins involving volatile chemicals requires a fully exhausted BSC or use of secondary containment (glove bag or box) inside a fume hood. Use of powdered toxins should be avoided if possible and minimized otherwise. o All manipulations with powdered toxins in open vials must be conducted in a Class III BSC (no secondary containment required) or in a Class II BSC or fume hood with the use of secondary containment (e.g., a disposable glove bag or box inside). o Respirator use may be required even for work inside a BSC for toxins with a low LD 50 or LC50 for protection in the event of engineering control failure. Operations that expose toxin solutions to vacuum or pressure should always be conducted within a certified fume hood or BSC o Respiratory protection may be required to protect personnel in case of engineering failure. Contact the Biological Safety Officer (BSO) for guidance on respiratory protection requirements. o If vacuum lines are used during toxin manipulations, they must be protected by an in-line HEPA filter to prevent contamination of the building vacuum system with toxin. 7. Safe Work Practices: If animals will be dosed with the toxin, the “Animal Use of Biotoxins” section (Appendix C) must be completed and followed. The Animal section includes requirements related to notification and coordination the Animal Care Services (ACS), room and cage labeling and health considerations for animal caretakers. Things to consider when listing Safe Work Practices for toxins (include all that apply): If powdered stocks are purchased, rehydration of the powder stock must occur in a certified BSC due to possible pressure changes that may generate aerosols through the vent opening. o Rehydrate the powder through the septum without opening the vial. o Care should be taken not to over-pressurize the vial. o Cover/shield the vial stopper with a kimwipe soaked in (list decontamination solution). o Use extreme care when handling sharps. Pressurized tubes containing toxins should only be opened in a certified BSC with a HEPA filter. Safety sharps devices and safety needles should be used to minimize risk of cuts or punctures. Refer to the Safety Engineered Sharps handout on the UF Biosafety webpage for examples and ordering information. Centrifugation of toxin solutions requires the use of sealed rotors or centrifuge safety cups. o Rotors and safety cups should be loaded and unloaded in the BSC. o Exterior surfaces of the primary containers should be wiped with decontamination solution prior to being placed in rotor or safety cups. o Exterior surfaces of rotors or safety cups should be wiped with decontamination solution prior to being removed from the BSC. o After use, centrifuge rotors or safety cups should be decontaminated and rinsed with water or 70% Ethanol. 8. Personal Protective Equipment (PPE): Describe additional PPE requirements for each task involving the toxin. Things to consider when listing PPE for toxins (include all that apply): If a toxin is suspended in a solvent, appropriate gloves should be worn to prevent penetration of the solvent. Solvents can act as carriers for the toxin resulting in dermal exposure if the solvent readily penetrates the glove. o Good references for chemical-glove compatibility include: Chemical Guide and Permeation Tables found on the EH&S website of Oklahoma State University Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 10 of 17 2/24/13 Showa Best Gloves Chemrest Chemical Guide If there is a potential risk of cut or puncture, cut- or puncture-resistant gloves should be worn. See the Safety-Engineered Sharps handout on the UF Biosafety Webpage for examples and ordering information. When conducting liquid transfers or other operations that pose a potential splash or droplet hazard in a Class II BSC or fume hood, safety goggles and disposable facemask or face shield should be worn. If working with dry forms of toxin that are subject to spread by electrostatic dispersal, static-free disposable gloves must be worn. Wetting and wiping down gloves and surfaces before beginning work can also help reduce static. Note on Respirators: Respirators are masks designed to protect the wearer from specific airborne hazards and are different from surgical masks, which protect the wearer only from splashes and are primarily intended to protect others from infectious aerosols exhaled by the wearer. Respirator use requires employee participation in the Respiratory Protection Program, which involves medical clearance and annual fit testing. o It is strongly recommended that all personnel working with toxins participate in the Respiratory Protection Program and be fit-tested for an N95 respirator; at a minimum, this will ensure that personnel are prepared to handle a spill cleanup if necessary. 9. Storage, Transportation and Shipping: Describe any additional controls per your toxin risk assessment and/or the recommendation of the Biosafety Office. 10. Decontamination and Waste Disposal: Describe the waste disposal procedures taking the following into consideration: Discarded needles/syringes and other sharps should be placed directly into properly labeled, punctureresistant sharps containers, and inactivated/decontaminated as soon as practical. Depending on the toxin, contaminated materials and toxin waste solutions may be inactivated by physical means (incineration or extensive autoclaving), or by chemical means (soaking in suitable decontamination solutions for a specified period of time). o To determine the appropriate physical and/or chemical means of inactivating your particular toxin, consult one or more of the following: University of Virginia Biotoxin Inactivation Table Tables 1 and 2 of the NIH/CDC’s BMBL Guidelines for Work with Toxins of Biological Origin The MSDS of your toxin Use the following sample protocols as a template for the waste disposal procedures for your toxin: Sample protocol for disposal of solid waste and re-useable labware contaminated with toxins that can be inactivated by autoclaving: o Contaminated, or potentially contaminated items (gloves, pipet tips, eppendorf tubes, paper towels, etc), are collected in a red biohazard-labeled autoclavable bag in the BSC in a durable, leak-proof container. At completion of the experiment, the bag is closed and sprayed with freshly prepared (insert decontamination solution, concentration) and allowed to sit for a minimum of 5 minutes before removing from the BSC and transport to the autoclave. Contaminated PPE should also be disposed of in a red biohazard-labeled autoclavable bag and autoclaved. o Autoclave the material using a biohazard cycle on the autoclave. Recommended cycle for heat-labile toxins: 121°C, 15psi for a minimum of 1 hr. o At the completion of the cycle, open the autoclave slowly taking care not to breathe in the steam; allow autoclave to vent before collecting the waste from the autoclave. o Place the autoclaved bag into the biological waste box for disposal as biowaste. Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 11 of 17 2/24/13 Sample protocol for disposal of solid waste contaminated with toxins that cannot be inactivated by autoclaving: o Contaminated, or potentially contaminated items (gloves, pipet tips, eppendorf tubes, paper towels, etc), are collected in a red biohazard-labeled bag in the BSC in a durable, leak-proof container. At completion of the experiment, the bag is closed and sprayed with freshly prepared (insert decontamination solution, concentration) and allowed to sit for a minimum of 5 minutes before removing from the BSC. Additional contaminated, or potentially contaminated PPE (e.g., disposable gowns) is also collected in a red, biohazard-labeled bag and closed at the completion of the experiment. Place closed bags into a biowaste box lined with a red, biohazard-labeled bag. These boxes are picked up by Stericycle for incineration. Sample protocol for decontamination/cleaning of re-useable labware contaminated with toxins that cannot be inactivated by autoclaving: o The use of reusable labware for work with toxins should be eliminated whenever possible and minimized if not possible. o Re-usable labware (e.g., glass tubes, glass beakers/flasks, etc) will be submerged in freshly prepared (insert decontamination solution, concentration) for a minimum of (insert time) to allow time for toxin inactivation. o After the allotted time, the decon solution from the labware soak must be retained, collected and transferred to a secondary, leak-proof container and sealed. Fill out a yellow hazardous (chemical) waste label indicating concentration of components in the waste bottle (including the amount and concentration of bleach) and affix to the bottle. Store the doubly-contained inactivated toxin in the designated hazardous waste accumulation area (fill in location). Do not store toxin waste for prolonged periods of time. Toxin waste should be picked up ASAP as a best management practice. To schedule a hazardous waste pick up call EH&S (392-8400) or schedule online. EH&S Hazardous Waste will not pickup toxin materials that have not been inactivated. o Wash the re-usable glassware with soap and water prior to re-use. 11. Inactivation/Disposal of Liquid Stocks: Describe any additional controls per your toxin risk assessment and/or the recommendation of the Biosafety Office. 12. Spill Response: Describe any additional controls and/or procedures per your toxin risk assessment and/or the recommendation of the Biosafety Office. 13. Exposure Management: Describe any additional controls and/or procedures per your toxin risk assessment and/or the recommendation of the Biosafety Office. 14. Personnel Training: Add any additional laboratory-specific training requirements to the statement already provided in the SOP template. For additional help in completing this SOP, contact the EH&S Biosafety Office at 392-1591. Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 12 of 17 2/24/13 Appendix A: Sample Toxin Log Book Setup: Toxin Name: _________________________________ Date Received: _______________________________ Source/Received From: _________________________ Form Received (Liquid, Powder): _________________ Total Amount Received (in mg): __________________ Concentration of Liquid Stock: ___________________ Total volume Liquid Stock: ______________________ Starting Amount (mg) Amount removed/used (mg) Acute Toxin SOP v.2 Date of Removal Purpose Removed by (Print name) Maintain hard copy in Laboratory Page 13 of 17 Signature Amount remaining (mg) 2/24/13 Appendix B: Toxin in Use Sign TOXIN IN USE DO NOT ENTER Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 14 of 17 2/24/13 Appendix C: Use of Biotoxins or Acute Toxins in Animals Standard Operation Procedures for Handling Animals Dosed with (Fill in Toxin Name). Note: This document is intended to be adapted to the lab as a site-specific document. Additional precautions or regulations may be required per risk assessment by the Biological Safety Office and/or Institutional Biosafety Committee and/or Responsible Official. Please add/subtract information as necessary to accurately reflect the procedures specific to your project and avoid confusion. A copy of this SOP should be provided to the Animal Care Services (ACS) and ACS staff should be provided with information/training on the hazards associated with working with (fill in toxin name), required practices and procedures and proper handling of bedding, cages and all other husbandry materials (e.g., carcass disposal) associated with these experiments. Use of all biotoxins in animals must be documented in a protocol approved by the UF Institutional Animal Care and Use Committee (IACUC). IACUC approval #: fill in Notification and Signage: When animals are dosed with (fill in toxin name), research personnel must clearly label the cages of the animals with a “Chemical Hazard” sticker indicating the agent, date introduced and date cleared (the date cleared will be determined by the project staff). For animals to require special care by research staff- place a “Special Care by PI” Card on the cage indicating what the research staff will be responsible for (e.g., cage changing, feeding, water, etc). o This card should include the name and phone number of the individual responsible for providing o care. o A Monthly Summary sheet must be completed by the researcher(s) documenting the husbandry care provided by the research staff. Personal Protective Equipment: All personnel must wear appropriate personal protective equipment when handling animals, cages and bedding. For handling animals and cages (at a minimum): disposable long-sleeved lab coat, shoe covers, hair covers, double gloves, surgical/dust masks, eye protection. For dumping bedding: the above listed PPE, plus an N95 respirator (with face shield, goggles, or safety glasses) must also be worn. Animal Innoculations: Preparation, Transport and Inoculation of Animals with toxin: o All toxin doses must be prepared in a certified biological safety cabinet (BSC) o If injecting the toxin: Load individual toxin doses into a single syringe; do not load multiple doses. Single-use, disposable luer-lok syringes should be used at a minimum. Safety syringe/needle combinations should be considered. o If preparing toxin doses in your laboratory and transporting to the animal facility for dosing: Individual toxin doses will be loaded into syringes inside a BSC. Each toxin loaded syringe should be placed in a conical tube and secured using parafilm for transport. Alternatively, a safety syringe/needle combination with a protective sheath that has a transport position can be ordered and used. Enclosed syringes/needles will then be placed in a secondary hard-walled, sealable container (e.g., Tupperware™) labeled with a Biohazard sticker, PI name and contact number. Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 15 of 17 2/24/13 o o The outside surface of the secondary container will be decontaminated with freshly prepared (insert decon solution, concentration) prior to removal from the BSC and transport to the animal facility. The buddy system will be used for all injections of toxin into animals. For i.p. injections of toxins that are hazardous at low doses, anesthetizing animals should be considered to minimize risk of self-inoculation. At a minimum, puncture resistant gloves will be worn underneath two pairs of disposable gloves for all injections. After injections, the needle/syringe will be immediately placed in an appropriate puncture-resistant sharps container. Sharps containers will be autoclaved immediately at the completion of the experiment. Animal Cage-Change Procedures: Engineering Controls: Animals dosed with (insert toxin name) must be maintained in microisolater cages with filter tops at (insert ABSL level). o Note: For animals too large to be housed in cages with microisolator lids or on ventilated racks, you must consult with the Biological Safety Officer in advance to determine appropriate engineering controls. Cages will be opened (including for cage-changing, animal care, or experiment-related reasons) in a (choose the appropriate control: BSC, ventilated cage changing station, or chemical fume hood). Work Practices: Contaminated, or potentially contaminated, bedding must be deactivated by the method listed in the Waste Disposal section below. Outer gloves must be changed at least every two (2) hours, when they become torn or obviously contaminated with excreta AND before handling animals in other experimental groups. All personnel will wash hands after removing gloves. Safety glasses, safety goggles, and reusable face shields will be decontaminated with freshly prepared (insert decon solution, concentration) followed by a water rinse after use and stored in a clean location for re-use. Biological safety cabinets will be surface-decontaminated after each use with freshly prepared (insert decon solution, concentration) followed by a water rinse. Cleaning will proceed from least to most contaminated area. Waste Disposal Procedures: Note: Research personnel must coordinate with the ACS Husbandry staff prior to the start of any experiment involving inoculation of animals with a biotoxin or acute toxin to ensure that the appropriate procedures are followed for cage changing and disposal of bedding and carcasses. Cages should be clearly marked to indicate any special handling procedure. For toxins that can be inactivated by autoclaving: o Cages (with bedding and feed) will be double-bagged in red, biohazard-labeled, autoclavable bags and taped shut. o Bags will be sprayed with freshly prepared (insert decon solution, concentration) and allowed to sit for a minimum of 5 minutes prior to removing from the BSC. o Autoclave cages promptly when de-populated. The recommended cycle for inactivating heat-labile toxins is: 121°C, 15psi for 1 hour. o Once autoclaved, bedding can be dumped according to standard practices and disposed of as general trash. o Animal carcasses will be placed in biohazard bags and incinerated. Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 16 of 17 2/24/13 o All disposable PPE will be discarded in a biohazard bag before leaving the work area and subsequently autoclaved. For toxins that cannot be inactivated by autoclaving: o Collect contaminated bedding and feed in red biohazard-labeled bags. Label the bag “Animal bedding for incineration.” o Place the bag into a biowaste box lined with another red biohazard-labeled bag and tape the box shut at the completion of the experiment to be picked up by Stericyle for incineration. o Label the box with a facility pre-printed biohazardous waste label. o Submerge cages and water bottles in freshly prepared (insert decon solution, concentration) for a minimum of 1 hr. Waste decon solution from the cage soak should be retained, collected and placed into a secondary durable, leak-proof container and sealed. Fill out a yellow hazardous (chemical) waste label indicating concentration of components in the waste bottle (including the amount and concentration of bleach) and affix to the bottle. Store the doubly-contained inactivated toxin in the designated hazardous waste accumulation area (fill in location). Do not store toxin waste for prolonged periods of time. Toxin waste should be picked up ASAP as a best management practice. To schedule a hazardous waste pick up call EH&S (392-8400) or schedule online. EH&S Hazardous Waste will not pickup toxin materials that have not been inactivated. Cages can then be washed according to standard procedures. o Animal carcasses will be placed in biohazard bags and incinerated. o Collect all disposable PPE in a biohazard bag before leaving the work area and package for incineration. Acute Toxin SOP v.2 Maintain hard copy in Laboratory Page 17 of 17 2/24/13