Challenge Background Silicon Anodes
advertisement

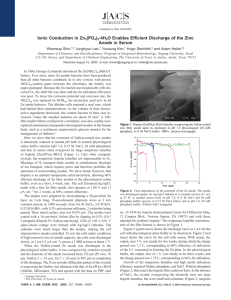
LIBs are widely used in applications ranging from small consumer electronics to electric vehicles to grid-scale electrical storage. Reliable, efficient and costeffective energy storage is essential if renewable power sources such as wind and solar are ever to reach their full potential. An LIB consists of a cathode composed of a lithium oxide, an electrolyte consisting of an organic solvent with a lithium compound dissolved in it, and an anode that is most frequently composed of graphite. During charging, lithium ions migrate to the anode and become embedded or intercalated in its crystal structure. During discharge, the current flows the other way, and lithium ions intercalate into the cathode. A silicon anode has tremendous advantages over a graphite one. Most importantly, it offers a tenfold improvement in charge density, which becomes particularly important when large-scale energy storage is considered. However, intercalation of lithium ions into a silicon anode produces stresses that are not a factor in graphite electrodes. These stresses eventually produce irreversible cracking in the anode, which degrades battery performance. The following image helps to visualize the process: www-ssrl.slac.stanford.edu Several technologies have been investigated to prevent or to mitigate cracking in silicon anodes, but none so far has applied to large batteries. Amprius has marketed nanotechnology-based silicon wire anodes that have been used successfully in cell phone batteries. They are hopeful that the technology could be scaled up to larger batteries.https://gigaom.com/2010/09/15/amprius-building-a-better-batteryfrom-the-anode-up/ Another area of research has been the use of a self-healing polymer that will fill cracks with a fully conductive material.http://www.nature.com/nchem/journal/v5/n12/extref/nchem.1802s1.pdf The following image illustrates how the self-healing polymer would correct the damage caused by lithium intercalation: power sources such as wind and solar are ever to reach their full potential. An LIB consists of a cathode composed of a lithium oxide, an electrolyte consisting of an organic solvent with a lithium compound dissolved in it, and an anode that is most frequently composed of graphite. During charging, lithium ions migrate to the anode and become embedded or intercalated in its crystal structure. During discharge, the current flows the other way, and lithium ions intercalate into the cathode. A silicon anode has tremendous advantages over a graphite one. Most importantly, it offers a tenfold improvement in charge density, which becomes particularly important when large-scale energy storage is considered. However, intercalation of lithium ions into a silicon anode produces stresses that are not a factor in graphite electrodes. These stresses eventually produce irreversible cracking in the anode, which degrades battery performance. The following image helps to visualize the process: www-ssrl.slac.stanford.edu Several technologies have been investigated to prevent or to mitigate cracking in silicon anodes, but none so far has applied to large batteries. Amprius has marketed nanotechnology-based silicon wire anodes that have been used successfully in cell phone batteries. They are hopeful that the technology could be scaled up to larger batteries.https://gigaom.com/2010/09/15/amprius-building-a-better-batteryfrom-the-anode-up/ Another area of research has been the use of a self-healing polymer that will fill cracks with a fully conductive material.http://www.nature.com/nchem/journal/v5/n12/extref/nchem.1802s1.pdf The following image illustrates how the self-healing polymer would correct the damage caused by lithium intercalation: www.engineering.com