Tests, Surveys, Interviews, or Passive Observations of Public Behavior
advertisement
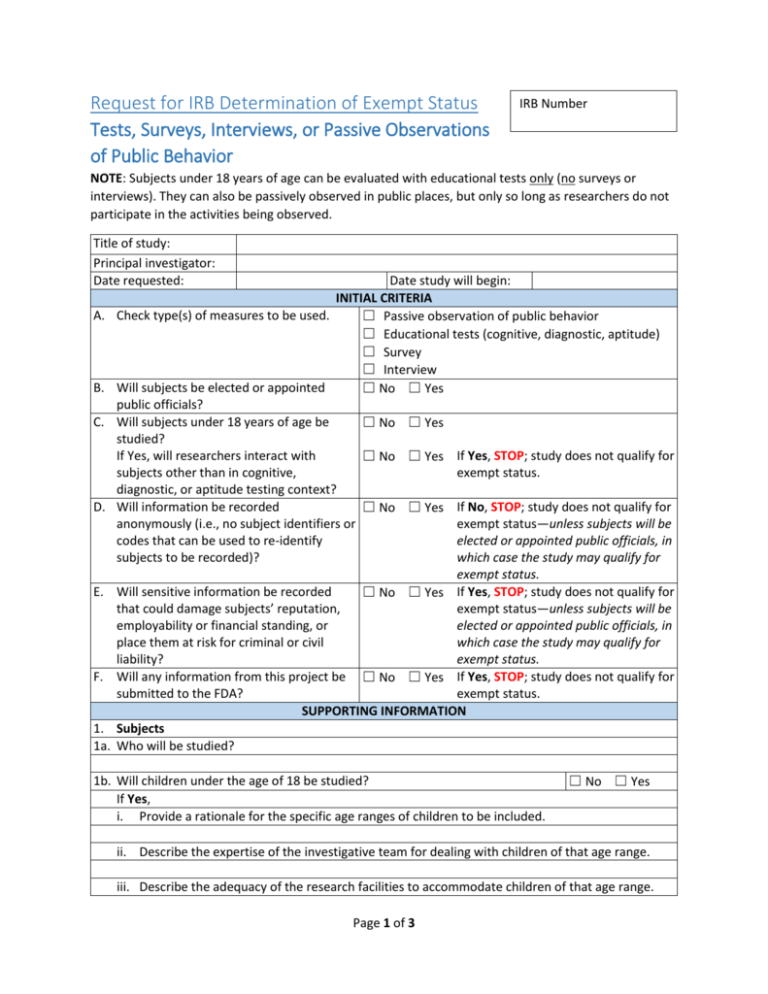
Request for IRB Determination of Exempt Status Tests, Surveys, Interviews, or Passive Observations of Public Behavior IRB Number NOTE: Subjects under 18 years of age can be evaluated with educational tests only (no surveys or interviews). They can also be passively observed in public places, but only so long as researchers do not participate in the activities being observed. Title of study: Principal investigator: Date requested: A. B. C. D. E. F. 1. 1a. Date study will begin: INITIAL CRITERIA Check type(s) of measures to be used. ☐ Passive observation of public behavior ☐ Educational tests (cognitive, diagnostic, aptitude) ☐ Survey ☐ Interview Will subjects be elected or appointed ☐ No ☐ Yes public officials? Will subjects under 18 years of age be ☐ No ☐ Yes studied? If Yes, will researchers interact with ☐ No ☐ Yes If Yes, STOP; study does not qualify for subjects other than in cognitive, exempt status. diagnostic, or aptitude testing context? Will information be recorded ☐ No ☐ Yes If No, STOP; study does not qualify for anonymously (i.e., no subject identifiers or exempt status—unless subjects will be codes that can be used to re-identify elected or appointed public officials, in subjects to be recorded)? which case the study may qualify for exempt status. Will sensitive information be recorded ☐ No ☐ Yes If Yes, STOP; study does not qualify for that could damage subjects’ reputation, exempt status—unless subjects will be employability or financial standing, or elected or appointed public officials, in place them at risk for criminal or civil which case the study may qualify for liability? exempt status. Will any information from this project be ☐ No ☐ Yes If Yes, STOP; study does not qualify for submitted to the FDA? exempt status. SUPPORTING INFORMATION Subjects Who will be studied? 1b. Will children under the age of 18 be studied? If Yes, i. Provide a rationale for the specific age ranges of children to be included. ☐ No ☐ Yes ii. Describe the expertise of the investigative team for dealing with children of that age range. iii. Describe the adequacy of the research facilities to accommodate children of that age range. Page 1 of 3 Request for IRB Determination of Exempt Status: Tests, Surveys, Interviews, or Passive Observations of Public Behavior, continued iv. Will sufficient numbers of children be studied to answer the scientific questions? Please elaborate. v. Is the research limited to educational tests or passive observations of ☐ No ☐ Yes public behavior? 1c. Will you be interacting with non-English speaking subjects? ☐ No ☐ Yes i. If Yes, are you fluent in the language they understand? ☐ No ☐ Yes ii. If No, indicate how you will communicate with subjects (e.g., with interpreter-if so, who will serve in that role. 2. Recruitment 2a. How will potential subjects be identified and how and where will they be approached for participation? 2b. Describe the recruitment materials (ads, letters, recruitment script, e-mails etc.) to be used, if applicable, and attach a copy of each. 2c. i. If applicable, attach the introductory script that describes the study and includes relevant elements of consent (see informed consent templates at X:\Institutional Review Board\Templates). ii. If NOT applicable, explain why. 3. Methods 3a. List the measures (e.g., surveys, questionnaires, etc.) to be used, and attach a copy of each. 3b. Describe how information will be obtained (e.g., face to face, phone, mail, Internet). 3c. Describe where study will be conducted, and who will collect data. 3d. Indicate how often subjects will be contacted and why. 3e. Describe how confidentiality of data will be protected and maintained. 3f. If subjects will be paid or otherwise compensated or incentivized, indicate how much they will receive, and how they will be compensated. 4. Analysis How will results be analyzed to determine that study aims have been met? 5. Additional Information, Clarification, or Comments for the IRB Reviewer CERTIFICATIONS AND ASSURANCES I am responsible for the conduct of this research protocol, including the co-investigator(s) and other research staff. I have certified that all co-investigator(s) and other research staff have completed the training requirements. I hereby certify that Page 2 of 3 Request for IRB Determination of Exempt Status: Tests, Surveys, Interviews, or Passive Observations of Public Behavior, continued The information contained in this document is accurate and correct. I will carefully follow the approved research protocol and submit all changes to the IRB for consideration before incorporating them into the study. I will notify the IRB of any deviations from the approved research protocol taken in an emergency to protect the subject from harm; and any unanticipated problem, or serious, unusual, or unanticipated adverse event. I or my designee will abide by the informed consent process with each subject and document this process on the approved consent form, allowing each subject adequate time before the study to decide voluntarily to participate in this study. I will protect the rights and welfare of each subject to the best of my ability. Principal Investigator’s Signature Date Page 3 of 3











