- Pacific Lutheran University

Request for Exempt Status (to Accompany HPRB Research Proposal)
Name(s):
If student researcher(s), name of Faculty Supervisor:
Project Title:
Note: Refer to the HPRB Policy Manual (see Chapter 3 IIA and IIB, pp. 4-5, and Chapter 5 IIIA, pp. 15-16).
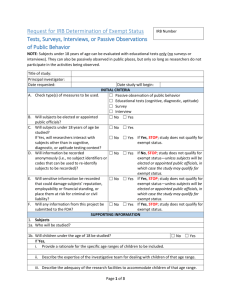
Part I: Federal Exemption Category ( Check the box for the category that you consider most applicable):
(1) Educational Practices: Normal educational practices are activities that occur regardless of whether the research is conducted.
Notes: (a) A study that evaluates a new instructional strategy or curriculum, or that randomly assigns students to different instructional strategies/curricula for comparison that would create inequity, would probably not be exempt since these are not “normal educational practices.” (b) Studies that involve surveys and interviews with minors that are outside the “normal educational practices” also do not qualify for this category of exemption.
(2) Educational Tests (Cognitive, Diagnostic, Aptitude, Achievement), Survey Procedures, Interview
Procedures, or Observation of Public Behavior Unless (a) Subjects can be identified, directly or through identifiers linked to the subjects, and (b) disclosure of subject’s responses outside the research could reasonably place the subject at risk of criminal or civil liability or be damaging to the subject’s financial standing, employability, or reputation . Notes: An anonymous workplace satisfaction survey or study involving interviews with college seniors (age 18 and older) about their plans after graduation could be exempt. (i) Research involving surveys or interviews with children, or observation of public behavior when investigators interact with children, does not qualify for exempt status under this category. (ii) Lack of identifiability means that the data are collected/recorded anonymously; there are no video recordings or photographs nor is it possible to identify a person when multiple pieces of information are brought together.
(3) Educational Tests as described in (2) above, except: Elected or appointed public officials or candidates for public office (e.g., mayors, governors, school superintendents, school board members, police chiefs) are public servants with a different privacy standard not requiring anonymity for data collection nor is there the same concern for risk due to disclosure. Notes: For example, an exempt study could involve interviews of town mayors about their religious beliefs and views on the separation of church and state or could include interviews of candidates for the state legislature about their finances and state employment.
(4) Existing Data or Specimens: The use of identifiable health care information for research use without the consent of the patients requires HPRB review, even when the research meets other criteria for exempt status. Notes: To meet the criteria for exemption within this category, already existing data must be abstracted so that it does not include direct identifiers (e.g., names, social security numbers, addresses, etc.) or indirect identifiers (e.g., codes or pseudonyms). HIPAA and state protected records cannot be reviewed as exempt.
(5) Federally-Supported Projects for the Public Benefit: Exemption based upon this category is extremely rare; see criteria and discuss your study with a UD and/or HPRB member.
(6) Taste and Food Quality Evaluation/Consumer Acceptance Studies : Concerns regarding exemption include, but are not limited to: (a) presence of additives or ingredients manipulated by the investigator, (b) volume of food to be consumed (i.e., reasonable eating behaviors), (c) agricultural chemical or environmental contaminants at or below the level found to be safe. Notes: An example of an exempt study could be a taste test on different varieties of a fruit to determine consumer preference when the fruits do not have any additives and subjects are asked to indicate which fruit they prefer. Studies involving consumption of alcohol, vitamins, and nutritional supplements do not qualify for exempt status.
Part II: Proposal Description. Describe how this proposal meets the criteria for the category above, including a clear explanation of how concerns for risk and privacy due to accidental disclosure have been eliminated.
Label this document using the same naming conventions as throughout your proposal. Identify this form as “ exempt-form
” and use “ exempt-description
” before the file extension (i.e., .doc or .pdf) for
Part II .
Pacific Lutheran University 2015-2016