Federal Categories of Review
advertisement

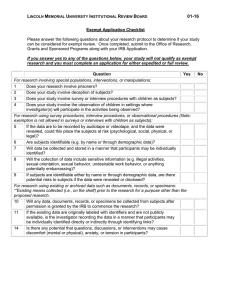
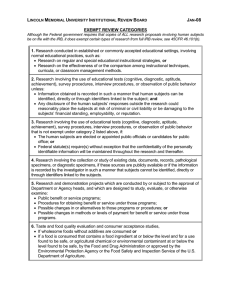
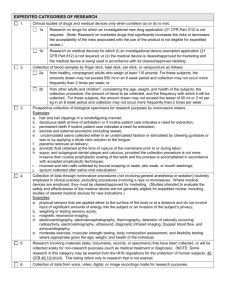
Federal Categories of Review Use this information to recommend categories of review for projects in your IRB application. Please remember that the IRB committee will decide the category of review based upon the information provided in your application, not on your recommendation. If you think your project qualifies for exempt status, please contact the IRB for authorization prior to starting the project. Any project not covered in the categories below will go for Full IRB Board Review. Expedited 1) No more than minimal risk – “The probability of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests”. 2) The entire project must be consistent with one or more of the following federally defined categories a. Clinical studies on drugs or medical devices for which an IND or and IDE application is not required. Similarly, a study with a cleared/approved medical device that is being used in accordance with its cleared/approved labeling. b. Collection of blood samples by finger stick, heel stick, ear stick or venipuncture. c. Prospective collection of biological specimens for research purposes by noninvasive means. d. Collection of data through noninvasive procedures routinely employed in clinical practice. Includes physical sensors, weighing, testing sensory acuity, MRI, ECG, EEG, EMG, ultrasound, and echocardiography. Excludes any procedure involving anesthesia or sedation, x-rays or microwaves, or use of unapproved medical devices. e. Research involving data, documents, records, or specimens that have been collected or will be collected solely for non-research purposes. Some research in this category may be exempt. f. Collection of data from voice, video, digital, or image recordings made for research purposes. g. Research on individual or group characteristics or behavior, or research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies. Some research in this category may be exempt. h. Continuing review of research previously approved by the IRB where no new subjects are being enrolled, all enrolled subjects have completed study activities, and research only remains active for long term follow up of subjects. Exempt 1) The following six categories are exempt from IRB regulations a. Research conducted in established or commonly accepted educational settings, involving normal educational practices. b. Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, or observation of public behavior. Some observation studies do not qualify for exemption, determined by level of risk and interaction with subjects. c. Research not exempt under b above, may qualify if subjects are elected or appointed public officials or candidates for public office. d. Research involving the collection or study of freely available de-identified existing data, documents, records, pathological specimens, or diagnostic specimens. e. Research and demonstration projects conducted by heads of government departments or agencies which are designed to evaluate public programs. f. Taste and food quality evaluation and consumer acceptance studies.