H NT Chapter 5, 6 & 7—Energy and the Periodic Table
advertisement
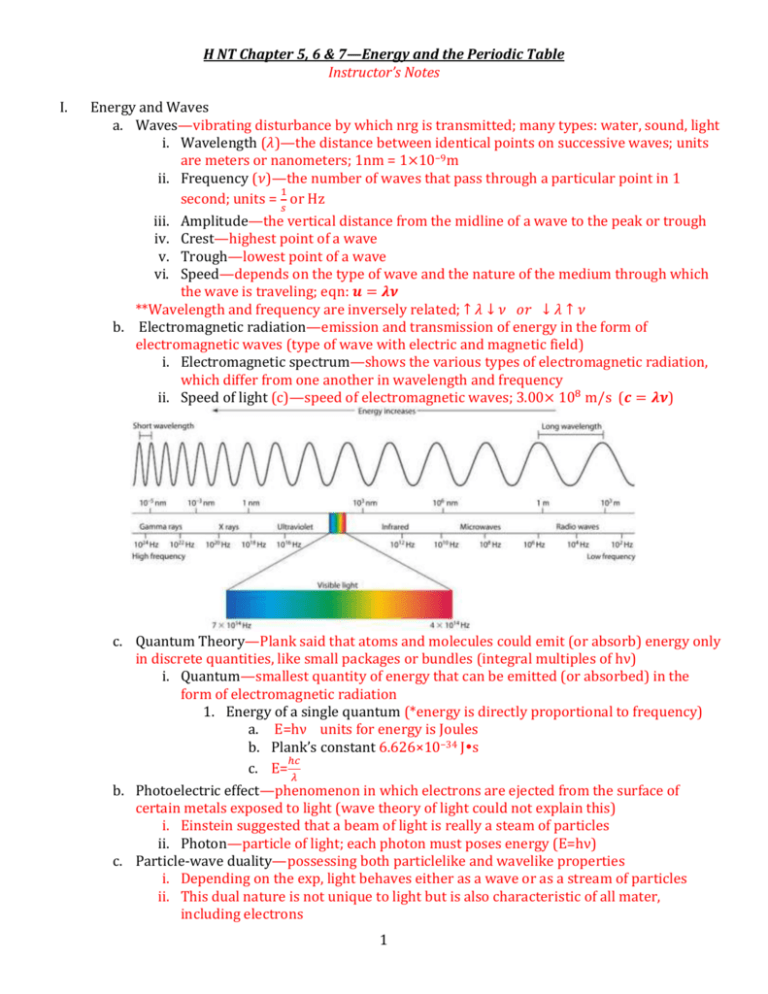
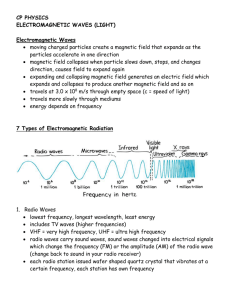
H NT Chapter 5, 6 & 7—Energy and the Periodic Table Instructor’s Notes I. Energy and Waves a. Waves—vibrating disturbance by which nrg is transmitted; many types: water, sound, light i. Wavelength (𝜆)—the distance between identical points on successive waves; units are meters or nanometers; 1nm = 1×10−9m ii. Frequency (𝜈)—the number of waves that pass through a particular point in 1 1 second; units = 𝑠 or Hz iii. Amplitude—the vertical distance from the midline of a wave to the peak or trough iv. Crest—highest point of a wave v. Trough—lowest point of a wave vi. Speed—depends on the type of wave and the nature of the medium through which the wave is traveling; eqn: 𝒖 = 𝝀𝝂 **Wavelength and frequency are inversely related; ↑ 𝜆 ↓ 𝜈 𝑜𝑟 ↓ 𝜆 ↑ 𝜈 b. Electromagnetic radiation—emission and transmission of energy in the form of electromagnetic waves (type of wave with electric and magnetic field) i. Electromagnetic spectrum—shows the various types of electromagnetic radiation, which differ from one another in wavelength and frequency ii. Speed of light (c)—speed of electromagnetic waves; 3.00× 108 m/s (𝒄 = 𝝀𝝂) c. Quantum Theory—Plank said that atoms and molecules could emit (or absorb) energy only in discrete quantities, like small packages or bundles (integral multiples of hν) i. Quantum—smallest quantity of energy that can be emitted (or absorbed) in the form of electromagnetic radiation 1. Energy of a single quantum (*energy is directly proportional to frequency) a. E=hν units for energy is Joules b. Plank’s constant 6.626×10−34 Js ℎ𝑐 c. E= 𝜆 b. Photoelectric effect—phenomenon in which electrons are ejected from the surface of certain metals exposed to light (wave theory of light could not explain this) i. Einstein suggested that a beam of light is really a steam of particles ii. Photon—particle of light; each photon must poses energy (E=hν) c. Particle-wave duality—possessing both particlelike and wavelike properties i. Depending on the exp, light behaves either as a wave or as a stream of particles ii. This dual nature is not unique to light but is also characteristic of all mater, including electrons 1 d. Bohr’s Theory i. Bohr’s Model—an atom was thought to be made of electrons and protons, with the electrons orbiting the nucleus in circular paths at high velocities *According to the laws of classical physics an electron in the hydrogen atom would experience acceleration toward the nucleus by radiating away nrg in the form of electromagnetic waves *Postulated that the electron is allowed to occupy only certain orbits of specific energies (energies of the electrons are quantized) *Bohr attributed the emission of radiation by an energized hydrogen atom to the electron dropping from a higher energy orbit to a lower one, emitting a quantum of energy (photon) in the form of light **one electron is capable of many different excited states 1. Ground state—lowest energy state of an atom 2. Excited state—higher in energy than the ground state ii. Spectroscope—instrument used to measure properties of light over a specific portion of the electromagnetic spectrum; prism/diffraction grating iii. Emission spectra—continuous or line spectra of radiation emitted by substances; can be seen by energizing a sample of material with some form of energy (thermal, electrical) 1. Continuous—all wavelengths of visible light are represented in the spectra; characteristic of the sun & heated solids 2. Bright-line—light emission only at specific wavelengths; characteristic of atoms in the gas phase (*every element has a unique emission spectrum and the characteristic lines an atomic spectra can by used in chemical analysis to identify unknown atoms, much as fingerprints are used to identify people) iv. Flame Test—way to determine the element present in a sample v. Neon signs—filled with different gases that are “excited” by electricity 2