Pure Substances - Elements and Compounds 2015
advertisement

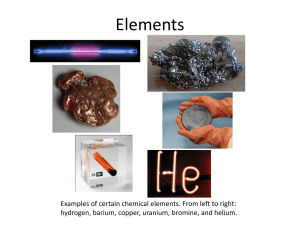
Pure Substances – Elements and Compounds Atoms are the most basic particle that make up everything in the universe. If a material is made of only one type of atom, we call it a pure substance. It will have certain properties by which it can be identified. No matter where a sample of a pure substance comes from, its properties will always be the same because it is made just from that one kind of atom. Examples of this type of pure substance are oxygen, hydrogen, carbon and gold. These pure substances made of only one type of atom are called elements. They are the most elementary, or most basic of pure substances. There are 92 naturally occurring elements in the universe. That’s it – just 92. Pure substances can be made of more than one kind of atom if, and only if, the different kinds of atoms are combined in identical sets. These sets of atoms bonded together are called molecules. For example, the formula H2O represents one molecule of water. Every molecule of water is identical. Even though it is made of more than one type of atom, water is a pure substance because all its molecules are the same. Because of this, any sample of water, no matter where in the world it comes from, will be exactly the same as long as it is just pure water with nothing mixed in it. Pure substances made of molecules that have more than one type of atom (like water) are called compounds. Kool-Aid is a mixture, not a pure substance. The difference between a mixture and a pure substance is that a pure substance is made of only one type of particle (either one type of atom or one type of molecule), and mixtures are made of different types of particles together in the same place. The particles off a mixture are not combined chemically in any particular ratio. They do not make identical sets like molecules; therefore, the different types of particles can be present in different amounts. If this is true, different samples of a mixture can have different properties. For example, sugar is a pure substance because all the molecules of sugar are exactly the same, and water is a pure substance because all its molecules are the same. But, if you mix sugar and water together, you will have a mixture containing two different kinds of particles. Before we even consider color and flavor, we know that Kool-Aid is not a pure substance because it has two different kinds of particle in it: water molecules and sugar molecules. pure substance: matter made of only one type of particle (one type atom or one type of molecule); or matter made of more than one type of atom, but the type of atoms are chemically combined in a set ratio element: a pure substance that cannot be broken down into a simpler substance by physical or chemical means; made of only one type of atom atom: the basic particle from which all elements are made compound: a pure substance made from two or more elements chemically combined in a set ratio molecule: two or more atoms bonded together (covalently); can be the same or different types of atoms; the most basic particle in from which compounds are made mixture: a combination of different substances ratio: relationship in amount between two different things 1. What is a pure substance? (A pure substance is….) 2. Why is oxygen considered a pure substance? (Oxygen is considered a pure substance because…) 3. What is an element? (An element is…) 4. How many elements exist naturally in the universe? (There are… naturally existing elements.) 5. When can a pure substance be made of more than one kind of atom? (A pure substance can be made of more than one kind of atom if…) 6. What is an example of a pure substance made of more than one element? (An example of a pure substance made of more than one element is…) 7. What is a compound? (A compound is…) 8. How are compounds different from elements? (Compounds… while elements….) 9. How are compounds and elements alike? (Compounds and elements are both made…) 10. Compounds and mixtures are both made of more than one thing. Why are compounds considered pure substances while mixtures are not? Give two reasons. (Compounds… while mixtures…; compounds… while mixtures…)