Activated Multi-Walled Carbon Nanotube supported Iron (III
advertisement

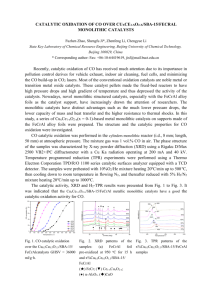
Activated Multi-Walled Carbon Nanotube supported Iron (III) Porphyrins as Efficient Recoverable Catalysts for Selective Aqueous Hydrocarbon Oxidation Atena Naeimi1, Abdolreza Rezaifard2, Maasoumeh Jafarpour2, Mohammad mahdi Doroodmand3 1 Department of Inorganic Chemistry, Universitat Autònoma de Barcelona, Barcelona, Spain Research Laboratory, Department of Chemistry, University of Birjan, Birjand, Iran. 3 Department of Chemistry, College of Science, Shiraz, 71454, Iran 2 Catalysis Atena.Bagheini@uab.cat, At_naimi@yahoo.com Abstaract The unique properties of carbon nanotubes (CNTs) have attracted the attention of researchers to use it in composite materials for a variety of applications, including electrostatic discharge, clean energy technology, drug delivery applications [1]. Also, CNTs are potential materials for the immobilization/encapsulation of active metallocomplexes based on their “unusual” structures and properties, [2]. The metalloporphyrins mimicking cytochrome P-450 enzyme, has been used vastly in last few decades due to important roles in both biochemistry processes such as hydrocarbon oxidation and different chemical transformations [3]. A problem often encountered in the porphyrin complexes catalyzed oxidation reactions is oxidative degradation of ligand and the difficulties in separating the products and contamination by residual catalyst [4]. A way to overcome this limitation is their immobilization/encapsulation into supports such as silica, cationic exchanger montmorillonite clay, silanized kaolinite and other matrices [5]. Selective catalytic materials may result from controlling the formation of either the pore structure of support or three dimensional network of the matrix [5]. In this context, the shape of CNTs offers interesting immobilization alternatives, which can takes place at the surface or inside the tubes, thus leading to highly active and selective oxidation catalysts [6]. In the present work, iron meso-tetraphenylporphyrins were supported on activated multi-walled carbon nanotube (AMWCNT) and were characterized by XRD, Raman spectroscopy, TGA, SEM and TEM. They were used in highly selective aqueous epoxidation of olefins to epoxide and oxidation of saturated hydrocarbons to the related carbonyl compounds as heterogeneous catalysts by tetra-nbutylammonium peroxomonosulfate (n-Bu4NHSO5) (Figure.1). Organic co-solvent, surfactants, co-catalyst and hydrophilic auxiliaries are unnecessary in this novel oxidation strategy. The catalyst could easily be recycled and reused without loss of activity and the oxidant’s by-product (n-Bu4NHSO4) could also be recycled. References: [1] H. Sharghi, M. H. Beyzavi, A. Safavi, M. M. Doroodmand, R. Khalifeh, Adv. Synth. Catal, 351 (2009) 2391-2410. [2] G. S. Machado, K. A. D. De Freitas Castro, F. Wypych, S. Nakagaki, J. Mol. Catal. A: Chem, 283 (2008) 99-107. [3] J. R. Lindsay Smith, Metalloporphyrins in Catalytic Oxidation, Marcel Dekker Inc., New York (1994) 325. [4] ) Q. –H. Fan, Y. –M. Li, A. S. C. Chan, Chem. Rev. 102 (2002) 3385-3466; [5] R. C. S. Luz, F. S. Damos, A. A. Tanaka, L. T. Kubota, Y. Gushikem, Talanta, 76 (2008) 1097-1104. [6] G. Pagona, A. S. D. Sandanayaka, Y. Araki, J. Fan, N. Tagmatarchis, G. Charalambidis, A. G. Coutsolelos, B. Boiitrel, M. Yudasaka, S. Iijima, O. Ito, Adv. Funct. Mater, 17 (2007) 1705-1711. Figures: Fig. 1. The oxygenation of hydrocarbons using AMWCNT/FeTPP_by n-Bu4NHSO5 in neat water Fig. 2. (A)SEM and (B) TEM images of FeTPP/AMWCNT