National Standards Chemistry Student (Bryan Proposal)
advertisement

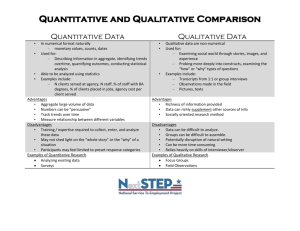
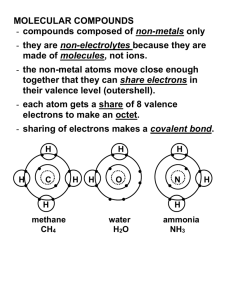
Academic Chemistry Standard LS 0: Students will safely identify and make reliable qualitative and quantitative measurements to describe the world. LS 1: Students will understand matter is composed of tiny particles called atoms and how atoms move and interact gives the properties of matter we observe. LS 2: Students will be able to describe how electrons move within an atom and how the arrangement of electrons within elements give rise to patterns on the periodic table. LS 3: Students can construct models of atomic bonding and describe how the electrons move with covalent and ionic bonding. LS 4: Students understand the language of a chemical reaction and can describe how elements are combined, pulled apart, and rearranged in a chemical reaction to produce more stable forms of matter. LS 5: Students will understand and apply the relationship of energy, kinetics, and equilibrium to a chemical reaction. LS 6: Students will be able to quantify a chemical reaction using dimensional analysis and the concept of a mole. LS 7: Students will be able to apply the kinetic molecular theory to gases both quantitatively and qualitatively. LS 8: Students will be able to describe the qualitative and quantitative properties of solutions including acids and bases. LS 0 & LS 5 will be incorporated throughout the year Performance indicators Students who demonstrate understanding can: R0a : Demonstrate proper safety. R0b: Demonstrate proper techniques to gather data R0c: Analyze and interpret data to draw conclusions R 1: classify matter and the changes it undergoes. R 3: understand that all matter is made up of particles called atoms which themselves are made up of subatomic particles. R 4a: understand how the electrons are arranged in the atom and can apply this concept to explain the properties of elements R4b understand how the periodic table is organized R 5: apply the concepts of transferring or sharing of electrons between atoms in order to form stable compounds. R 6: name and write formulas for covalent and ionic compounds. R 9a: apply the law of conservation of mass as it pertains to chemical reactions. R9b: describe how elements and compounds undergo changes during chemical or nuclear reactions R 14a: understand and apply the relationship of energy as it relates to states of matter and chemical reactions R14b: understand and apply the concept of kinetics as it relates to chemical reactions R14c: understand and apply the concept of equilibrium as it relates to a chemical reaction. R 7: convert units using dimensional analysis R 8: analyze the chemical composition of a substance using the concept of a mole. R 10: quantify a chemical reaction using stoichiometry. R 11a: describe the kinetic molecular theory of matter. R11b: quantify the relationship between the Kelvin temperature, pressure, volume, and moles of a gas. R 12: apply the qualitative and quantitative properties of solutions. R 13: apply the qualitative and quantitative properties of acids and bases. Two pieces of evidence (graded assessments) per performance indicator Performance Indicators are to be the same weight. LS’s can be weighted differently. What separates 4-ness & 3-ness? … Connecting old material to new material is a difference. The rubric can be used to separate 4’s from 3’s so you don’t necessarily need some idea as a performance indicator.