grl53199-sup-0001-supinfo
advertisement

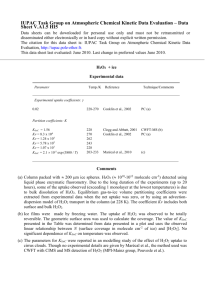
Geophysical Research Letters Supporting Information for HCFC-133a (CF3CH2Cl): OH Rate Coefficient, UV Absorption Spectrum, and Atmospheric Implications Max R. McGillen,1,2 François Bernard,1,2 Eric L. Fleming,3,4 and James B. Burkholder,1 1 2 Earth System Research Laboratory, Chemical Sciences Division, National Oceanic and Atmospheric Administration, Boulder, Colorado, USA. Cooperative Institute for Research in Environmental Sciences, University of Colorado, Boulder, Colorado, USA. 3 NASA Goddard Space Flight Center, Greenbelt, Maryland, USA. 4 Science Systems and Applications, Inc., Lanham, Maryland, USA. Contents of this file Figure S1 Tables S1 to S5 Additional Supporting Information (Files uploaded separately) Caption for Datasets S1 Introduction This supporting information includes tabulated data used to prepare Figures 1 and 2 in the manuscript (i.e. rate coefficient data, experimental conditions and UV cross section data), which is intended for quantitative use or comparisons with any future work. Also included is the infrared spectrum of HCFC-133a, which was used for calculating radiative efficiency. 1 Figure S1. Infrared spectrum of HCFC-133a (CF3CH2Cl). 2 Table S1. [OH]0c [HCFC-133a]d k' range (s-1) k(T)e 5.56 Photolysis Laser Fluenceb 1.9 0.3 0.79–14.2 320–760 0.34 ± 0.03 H2O2 6.68 1.9 2.9 0.86–10.1 165–730 0.61 ± 0.02 8.6 H2O2 25.4 1.5 8.5 1.41–12.0 415–1060 0.56 ± 0.05 9.6 H2O2 15.9 1.5 5.4 0.79–10.2 T (K) P (Torr) v (cm s-1) Photolysis Precursor 233 103 7.8 (CH3)3COOH 253 108 7.3 253 201 253 88 [Source]a 300–850 0.60 ± 0.05 Global k(253 K) 0.59 ± 0.01 274 111 8.1 H2O2 4.83 1.9 2.1 0.86–8.79 150–790 0.81 ± 0.04 296 101 10.9 H2O2 5.09 0.8 0.9 0.65–7.01 140–750 1.00 ± 0.08 296 92 9.6 (CH3)3COOH 11.7 1.5 0.4 0.61–7.55 490–1160 1.00 ± 0.06 296 94 10.5 H2O2 16.4 1.5 5.5 0.57–6.84 335–1000 1.11 ± 0.07 Global k(296 K) 1.10 ± 0.02 375–1470 1.62 ± 0.06 324 100 11.9 H2O2 352 93 13.9 H2O2 15.3 352 198 6.2 H2O2 11.8 379 93 14.1 H2O2 379 52 22.3 379 199 7.0 15.8 1.3 4.6 0.56–5.55 1.3 4.5 0.52–4.76 380–1730 2.23 ± 0.05 1.6 4.2 1.49–7.38 550–2060 2.34 ± 0.03 Global k(352 K) 2.30 ± 0.03 350–2160 3.07 ± 0.09 12.6 1.3 3.7 0.47–4.71 H2O2 21.3 1.6 7.7 0.31–3.97 505–1570 2.86 ± 0.09 H2O2 8.99 1.6 3.2 0.98–6.61 460–2290 3.23 ± 0.05 Global k(379 K) 3.10 ± 0.03 a units of 1013 molecule cm-3, b units of mJ cm-2 pulse-1, c units of 1010 molecule cm-3 [OH]0 = Precursor cross section at 248 nm x Precursor OH quantum yield x Laser Fluence, d units of 1016 molecule cm-3, e units of 10-14 cm3 molecule-1 s-1 and quoted uncertainties are 2σ measurement precision. Table S1. Experimental conditions and obtained rate coefficients for the OH + CF3H2Cl (HCFC-133a) reaction 1 Table S2. σ(λ,T) (10-22 cm2 molecule-1)a Wavelength (nm) 323 K 294 K – 184.95 601 ± 25 192.5 319 ± 2 304 ± 8 195 216 ± 2 197.3 ± 0.4 197.5 139.4 ± 0.8 128.7 ± 0.7 200 91.9 ± 0.7 83.8 ± 0.3 202.5 60.4 ± 1.2 53.5 ± 0.3 205 38.8 ± 0.5 33.3 ± 0.3 207.5 24.9 ± 0.2 21.2 ± 0.2 210 15.9 ± 0.1 13.32 ± 0.09 215 7.09 ± 0.05 5.93 ± 0.04 220 3.51 ± 0.04 2.93 ± 0.02 225 1.93 ± 0.04 1.545 ± 0.009 230 0.957 ± 0.019 0.768 ± 0.008 235 0.447 ± 0.022 0.370 ± 0.008 240 0.221 ± 0.016 0.164 ± 0.004 a Quoted uncertainties are 2σ measurement precision. but was included to represent the precision of the fit. 273 K 253 K 233 K 213 K – – – – 293 ± 2 271 ± 3 265.0 ± 0.6 262 ± 2 185 ± 1 176 ± 5 166.3 ± 0.8 163 ± 1 121 ± 1 111 ± 2 106.3 ± 0.8 102.8 ± 0.4 76.3 ± 0.7 70.9 ± 0.4 68.3 ± 1.2 65.6 ± 0.5 48.4 ± 0.4 44.4 ± 0.5 43.0 ± 0.3 40.9 ± 0.3 31.0 ± 0.3 28.3 ± 0.2 26.7 ± 0.5 25.2 ± 0.3 18.9 ± 0.3 17.3 ± 0.2 16.2 ± 0.1 15.1 ± 0.3 12.23 ± 0.04 11.1 ± 0.3 10.1 ± 0.2 9.3 ± 0.3 5.07 ± 0.09 4.89 ± 0.08 4.83 ± 0.08 4.0 ± 0.1 2.49 ± 0.03 2.46 ± 0.21 2.42 ± 0.07 2.2 ± 0.2 – 1.370 ± 0.013 1.28 ± 0.06 1.47 ± 0.09 – – 0.708 ± 0.010 0.618 ± 0.014 – – 0.323 ± 0.011 0.290 ± 0.015 – – – 0.131 ± 0.014 In some cases, the least significant digit may not be statistically significant, Table S2. UV absorption cross sections, σ(λ,T), for CF3H2Cl (HCFC-133a) measured in this work 2 Table S3. wavenumber (cm-1) 102.072 180.483 343.569 349.727 519.766 523.553 625.256 787.342 848.642 898.716 1108.06 1153.00 1258.93 1284.93 1343.84 1452.43 3052.12 3121.67 intensity (cm molecule-1) 4.65 × 10-19 2.11 × 10-19 1.81 × 10-19 7.94 × 10-20 2.70 × 10-19 7.96 × 10-19 3.91 × 10-18 3.76 × 10-18 4.08 × 10-18 1.58 × 10-18 1.73 × 10-17 3.99 × 10-17 1.77 × 10-17 2.37 × 10-17 1.56 × 10-17 3.72 × 10-18 1.22 × 10-18 5.48 × 10-21 Table S3. Calculated infrared frequencies made at MP2-6-311G(2d,p), no wavenumber scaling 1 Table S4. Integrated Band Strength (cm molecule-1) a Integration This worka Etminan et al., 2014 Range (cm-1) 780–920 (9.08 ± 0.02) × 10-18 9.02 × 10-18 1070–1530 (1.16 ± 0.003) × 10-16 1.12 × 10-16 -18 2970–3010 (1.14 ± 0.003) × 10 – Quoted uncertainties are 2σ of the measurement precision. Table S4. Infrared integrated band strengths, S, for CF3CH2Cl at 298 K from this work (spectrum given in Dataset S1) and the literature. Table S5: Lifetime (years) Radiative efficiency (Wm-2ppb-1) GWP (20 years) GWP (100 years) GWP (500 years) This work 4.45 0.155a 1317c 378c 115c Etminan et al. [2014] 4.3 0.15b 1220 340 96 a Calculated using a parameterized method reported in Hodnebrog et al. [2012]. radiative transfer model (NBM). c GWPs are for well-mixed gases. The difference between our GWP values and those of Etminan et al. [2014] is primarily due to our slightly higher radiative efficiency and longer lifetime. b Calculated using a narrow-band Table S5: Atmospheric lifetime and radiative efficiency of HCFC-133a and its GWP over the 20, 100 and 500 year time horizons. The values from the present work are compared with those reported by Etminan et al. [2014]. Data Set S1. Infrared spectrum data for HCFC-133a, provided as wavenumber and cross section (cm2 molecule-1 [base e]) 2