Physical & Chemical Properties Worksheet
advertisement

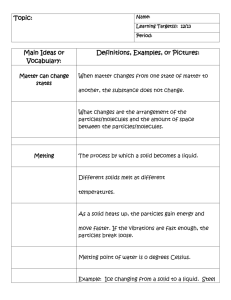
Physical and Chemical Properties Chemistry is the study of matter. Matter is anything that takes up space. When you look at a substance you can determine its PHYSICAL PROPERTIES by using your senses. This can be things like its colour, smell, and texture (We never use taste since it is dangerous). Another physical property is the state of matter (solid, liquid or gas). The state of matter is dependent on the speed of the particles that make up the substance. Slow moving particles tend to stay together to form a solid. Increasing the speed of the particles makes them move around more to create a liquid. Even faster particles spread out farther to create a gas. The CHEMICAL PROPERTIES of a substance are based on what reactions it could undergo. This is where you must use your knowledge of the substance. Some examples of chemical properties are combustion (also called flammable), rusting, tarnishing or reacting with acid. Physical vs. Chemical Properties 1. Use the information provided on pages 16-18 to fill in the following table. Table 1: Properties of Matter Property Meaning Examples Hardness Malleability Ductility Melting & Boiling Points Crystal Form Solubility Viscosity Density 2. How do you know that the properties defined in the table were all physical and chemical? not 3. For each of the following indicate if it is a physical or chemical property by checking off the correct column. Table 2: Physical or chemical? Physical Chemical red colour density flammability dissolves in water reacts with acid to make hydrogen explodes when heated bitter taste melting point reacts with water to form a gas reacts with a base to form water hardness shiny smells like rotten eggs 4. Fill in the blanks with the appropriate vocabulary that you learned today! A. When you describe a substance you look at its characteristics or _________________ B. When you say whether a substance is a solid, liquid or gas you are describing the ________________________________ C. The temperature at which something changes from a solid to a liquid is its ___________________ D. The temperature at which something changes from a liquid to a gas is its ______________________ E. If a solid mixes into a liquid so well that you can’t see it anymore we say the solid ____________________ F. How well a liquid flows is also called its __________________ G. A millilitre of lead has more mass then a millilitre of Styrofoam. The lead has a greater _____________ H. ________________ describes a material that can be bent or shaped easily.