Ch. 11 Sections 1 and 2 Study Guide Section 1 pgs. 455
advertisement

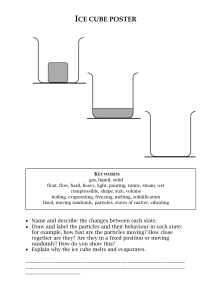
Ch. 11 Sections 1 and 2 Study Guide Section 1 pgs. 455-462 and notes Vocabulary-matter, mass, volume, density Examples of matter and 3 specific examples that are not matter. Why are these 3 things not matter? What unit do we measure mass? When we measure mass, we are finding out how much ________________ something has. What tools do we use to measure mass? Think about our experiments. What would have more mass an object with a lot of particles or a smaller amount of particles? What do unit do we use to find volume of a liquid? What’s the unit for the volume of a 3D object measured in cm? What would have more volume a balloon that’s blown up or a deflated balloon? How do we measure density? What units can we use? What does density tell us about an object’s particles that mass does not? What happens in water if something has a high density? What about a low density? What do particles look like for objects with a high and low density? What are physical properties? Which properties can be measured? Which ones can we only observe and not measure? Section 2 pgs. 464-472 Vocab-state of matter, solid, liquid, gas What are the 3 states of matter and some examples? What do particles look like in each state of matter? How are the particles moving in each state of matter? How does heat affect particles in matter? What happens if heat is added to an ice cube? What happens when heat is added to a liquid such as water? What does water become when it turns into a gas? Describe how an ice cube can change to a liquid and then gas. How can water vapor turn back into an ice cube? What’s the difference between a melting and boiling point?