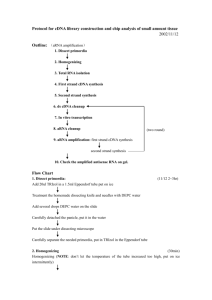
Protocol

RNA Amplification using full volumes of the MessageAmp TM II aRNA Kit
A. Reverse Transcription to Synthesise First Strand cDNA
1.
Add 1 l T7 Oligo (dT) Primer to a PCR tube.
2.
Add 11 l RNA, vortex briefly and pulse spin.
3.
Incubate samples at 70 C for 10 minutes in a thermal cycler.
4.
Centrifuge samples at 4 C briefly and place on ice.
5.
Prepare Reverse Transcription Master Mix at room temperature in the order below:
Component
10X First Strand Buffer
Amount per Sample
2 l
For 12 Samples
28 l dNTP Mix 4 l 56 l
RNase Inhibitor
ArrayScript
1 l
1 l
Vortex, centrifuge briefly at 4 C and place on ice.
14 l
14 l
6.
Add 8 l of Reverse Transcription Master Mix to each sample, mix by pipetting 2-3 times, flick the tube 3-4 times and spin briefly.
7.
Incubate reactions at 42 C for 2 hours in a thermal cycler.
8.
Centrifuge briefly at 4 C and place samples on ice.
B. Second Strand cDNA Synthesis
1.
Prepare Second Strand Master Mix on ice in the order below:
Component
DEPC Water
Amount per Sample
63 l
For 12 Samples
819 l
10X Second Strand Buffer dNTP Mix
10 l
4 l
2 l
130 l
52 l
26 l DNA Polymerase
RNase H 1 l
Vortex, centrifuge briefly at 4 C and place on ice.
13 l
2.
Add 80 l Second Strand Master Mix to each sample, mix by pipetting 2-3 times, flick the tube 3-4 times and spin briefly.
3.
Incubate samples at 16 C for 2 hours in a thermal cycler, leave the lid open.
4.
Place reactions on ice.
C. cDNA Purification
1.
Add 250 l of cDNA Binding Buffer and mix by pipetting.
2.
Pipet the sample onto the centre of a cDNA Filter Cartridge.
3.
Centrifuge for 1 minute at 10,000 rpm at room temperature, discard the flow through and replace the Filter Cartridge in the wash tube.
4.
Add 500 l of Wash Buffer (ensure 100% ethanol has been added) to the Filter Cartidge.
5.
Centrifuge for 1 minute, 10,000 rpm at room temperature, discard the flow though and centrifuge for a further 1 minute at 10,000 rpm.
6.
Transfer the Filter Cartridge to a cDNA Elution Tube and elute with 20 l nuclease free water
(pre-heated to 50 – 55 C). NB. If testing cDNA concentration after the purification step, elute with 24 l water.
7.
Leave at room temperature for 2 minutes, then centrifuge for 1.5 minutes at 10,000 rpm.
8.
Re-elute with the eluate.
D. In Vitro Transcription to Synthesise aRNA
1.
Prepare an IVT Master Mix in the order below at room temperature:
Component
T7 ATP Soln (75mM)
Amount per Sample
4 l
For 12 Samples
54 l
T7 CTP Soln (75mM)
T7 GTP Soln (75mM)
4 l
4 l
4 l
54 l
54 l
54 l T7 UTP Soln (75mM)
T7 10X Reaction Buffer
T7 Enzyme Mix
4 l
4 l
54 l
54 l
Vortex and centrifuge briefly.
2.
Add 24 l IVT Master Mix to each sample, mix by pipetting 2-3 times, flick the tube 3-4 times and spin briefly.
3.
Incubate the tubes at 37 C in an oven for 14 hours.
4.
Add 60 l DEPC water to each sample and vortex to mix.
E. aRNA Purification
1.
Add 350 l of aRNA Binding Buffer to each sample.
2.
Immediately add 250 l of 100 % ethanol, mix by pipetting and transfer to an aRNA Filter
Cartridge.
3.
Centrifuge for 1 minute, 10,000 rpm and discard the flow through.
4.
Add 650 l Wash Buffer, centrifuge for 1 minute, 10,000 rpm and discard the flow through.
Centrifuge for a further 1minute, 10,000 rpm and transfer the Filter Cartridge to an aRNA
Collection Tube.
5.
Elute with 50 l nuclease free water (preheated to 50 – 55 C).
6.
Leave at room temperature for 2 minutes and then centrifuge for 1.5 minutes, 10,000 rpm.
7.
Re-elute with the eluate.
8.
Nanodrop and run on Bioanalyzer to assess RNA integrity.