Unit 6 Lab 2 and 3 Write Up
advertisement

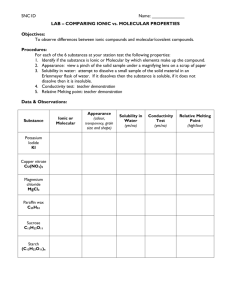
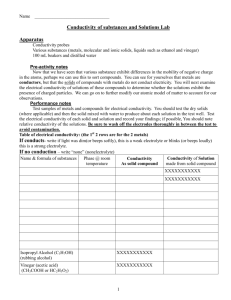
Name Veritas ________ Unit 6 – Lab 2 and 3 Combined Report Title Lab 2: Lab 3: Purpose (Complete sentences required.) Lab 2: Lab 3: Hypotheses: (Remember: It is okay to have different a hypothesis for substances within one of the categories. Explain substance-bysubstance within that category what you thought would happen and why. All hypotheses require an explanation/justification.) Lab 2. Conductivity of Metals: Conductivity of Solid Compounds: Conductivity of Dissolved Solid Compounds: Conductivity of various liquid compounds: Lab 3. Crystal structures of elemental metals v. elemental nonmetals v. ionic compounds v. molecular compounds: Procedures: Lab 2. (Insert numbered list of procedures here.) Lab 3. (Insert numbered list of procedures here.) Data: Lab 2: Strong Electrolytes Weak Electrolytes Non-electrolytes Lab 3: Atomic Solid Crystal Structures: (Cut out and tape the images below.) Atomic Solid Observations: (Write a bulleted list of observations here.) Molecular Solid Crystal Structures: (Cut out and tape the images here.) Ionic Solid Crystal Structures: (Cut out and tape the images here.) Molecular Solid Observations: (Write a bulleted list of observations here.) Ionic Solid Observations: (Write a bulleted list of observations here.) Analysis and Conclusion: (Complete sentences required for explanations.) Lab 1: Describe and explain the behavior observed in metals. Use a drawing to aid in your explanation. What type of bond is involved in metals? _________________________________________________ Would the same behavior be seen in non-metals? Explain why or why not. Use a drawing to aid in your explanation. What type of bond is involved in non-metals? ____________________________________________ Describe and explain the two types of behavior observed in compounds. Use drawings to aid in your explanation. Make sure to identify the type of bond involved in both groups of compounds. Lab 2: Develop a set of “Rules for Identification” of the 3 groups of solid substances. (Your rules must allow for correct classification of any substance. Identify the types of particles involved. Complete sentences required.) Atomic Solids: ________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ Molecular Solids: ____________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ Ionic Solids: __________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ Overall: (Using complete sentences, discuss the connections between the results of the conductivity lab and your observations from the crystal structures lab.)