Elements Ordered By Their Sub-Shell - Meta
advertisement

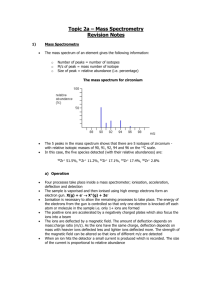
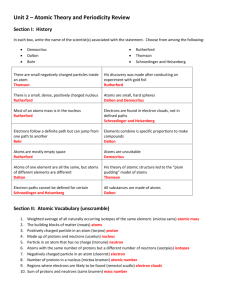
The Simplified Periodic Table: Elements Ordered By Their Sub-Shell Ben Alper Cranfield University, Bedfordshire, MK43 0AL, ben_alper@live.com Abstract: This article introduces a periodic table in which the elements are ordered by the energy level of their sub shells and by the number of electrons in their outer shell. Such a layout is both representative of the structure of atoms and has utility since it is easy to use. This article also introduces electron ’promotion’ which is a simple concept for characterising the structure of elements which do not conform to the Aufbau principle. Development of the periodic table Dmitri Mendeleev created the forerunner of the traditional periodic table 140 years ago in 1869. The elements were ordered by atomic weight and arranged so that elements with similar repeating properties were aligned in columns. Unlike other tables, Mendeleev’s was able to predict the weight and properties of new elements. In 1919 Henry Moseley reordered some elements in order of atomic charge rather than atomic weight: swapping Argon with Potassium, and putting Cobalt before Nickel. In 1945 during the Manhattan project, as Glenn Seaborg synthesised new elements he noticed that the Actinide group had different properties to the transition metals. He removed it and put it in a separate block along with the Lanthanide group resulting in the modern version of the traditional periodic table. Quantum theory of electron sub shells In 1923 Bohr proposed that the periodicity of the periodic table could be explained if the electrons (or quanta) orbiting the nucleus were ordered in shells. This was corrected to include sub shells by Stoner & Summerfield in 1924, and was further corrected by Pauli 1925, Schrodinger in 1926 and finally Madelung in1936. Despite the advances in understanding of atomic structure, the periodic table was left unchanged, perhaps because no one could be sure that the most recent change to the sub-shell structure would be the last. Even now, there is uncertainty over whether experimental evidence really proves the order of the sub shells. However there have been no major changes to the consensus on sub-shell structure in the last 70 years making sudden new developments less likely. The order of electron sub shells There are 4 types of sub shell known as s, p, d and f. S sub shells contain up to 2 electrons; p sub shells contain up to 6 electrons; d sub shells contain up to 10 electrons and f sub shells contain up to 14 electrons. Prefixing each sub shell type is the number of the shell known as the ‘principle quantum number’ thus the full name of a sub shell is written as e.g. ‘1s’. The naming convention of the principle quantum number is an artefact of the outdated Bohr model of the atom and can be misleading since the higher sub shells in a Bohr shell overlap the lower sub shells of the next shell, as shown in figure 2. The table below shows the order in which the sub shells are filled for the first 36 elements. Element Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Symbol H He Li Be B C N O F Ne Na Mg No 1 1 3 4 5 6 7 8 9 10 11 12 Electron Configuration 1s1 1s2 1s2 2s1 1s2 2s2 1s2 2s2 2p1 1s2 2s2 2p2 1s2 2s2 2p3 1s2 2s2 2p4 1s2 2s2 2p5 1s2 2s2 2p6 1s2 2s2 2p6 3s1 1s2 2s2 2p6 3s2 Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton Al Si P S Cl Ar K Ca Sc Ta V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 1s2 2s2 2p6 3s2 3p1 1s2 2s2 2p6 3s2 3p2 1s2 2s2 2p6 3s2 3p3 1s2 2s2 2p6 3s2 3p4 1s2 2s2 2p6 3s2 3p5 1s2 2s2 2p6 3s2 3p6 1s2 2s2 2p6 3s2 3p6 4s1 1s2 2s2 2p6 3s2 3p6 4s2 1s2 2s2 2p6 3s2 3p6 3d1 4s2 1s2 2s2 2p6 3s2 3p6 3d2 4s2 1s2 2s2 2p6 3s2 3p6 3d3 4s2 1s2 2s2 2p6 3s2 3p6 3d5 4s1 1s2 2s2 2p6 3s2 3p6 3d5 4s2 1s2 2s2 2p6 3s2 3p6 3d6 4s2 1s2 2s2 2p6 3s2 3p6 3d7 4s2 1s2 2s2 2p6 3s2 3p6 3d8 4s2 1s2 2s2 2p6 3s2 3p6 3d10 4s1 1s2 2s2 2p6 3s2 3p6 3d10 4s2 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p1 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p3 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p4 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 Table 1: The order in which electron shells are filled for the first 36 atoms. With a small number of exceptions, the electron shells of each element are filled in the same way as for the element before it, but with an extra electron in the outermost sub shell. This is known as the Aufbau principle. When a sub shell is filled, then the next outermost shell begins to be filled, as shown in figure 1. The order in which the sub shells are filled goes in order of the energy level of the sub shell, rather than the shell number. For example the 4s electron sub-shell is filled before the 3d sub-shell. The order of sub-shell energy levels can be seen in figure 2. D sub-shells have similar energy levels to the F sub-shell of the shell below and there is in fact some overlapping of the 5f and 6d sub shells. The number of elements with electron positions filled in an unexpected order is 19 out of 120 elements. However, in general the electrons positions are filled as expected, and the order in which the sub-shells are filled are as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 5f, 5d, 6p, 7s, 5f, 6d, 7p, 8s. From table 1, it can be seen that elements such as Chromium and Copper do not follow the usual pattern of the Aufbau principle. Their unusual configuration is highlighted in bold in table 1. The change in order of the electrons can be described by ‘electron promotion’ by which an element in a lower sub shell transfers to higher sub shell as the lowest energy state for that element. Figure 1: Filled electrons states for a phosphorus atom Elements ordered by their outer sub shell In the ‘Quantum table of the elements’; shown in figure 5, along the x axis, the elements are ordered by the energy levels of their outer sub shell. The number of electrons in the outer sub shell of each element are found down the y axis. The number of electrons in the outer sub shell affects the properties of the elements. Atoms with full outer sub shells are in a lower energy state than atoms with partially filled sub shells. In order to reach this lower energy state atoms are driven to bond with each other through ionic or valence bonding. As an example of this, the Noble gasses are un-reactive as they have full outer sub shells. Figure 2: Energy levels of the sub shells. Energy Level 6d Sub-Shell 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 1 H Li B Na Al K Sc Ga Rb Y In Cs La Lu Ti Fr Ac Lr 1 3 5 11 13 19 21 31 37 39 49 55 57 71 81 87 89 103 113 119 2 He Be C Mg Si Ca Ti Ge Sr Zr Sn Ba Ce Hf Pb Ra Th Rf Uuq Ubn 2 4 6 12 14 20 22 32 38 40 50 56 58 72 82 88 90 104 114 120 Uut Uue 3 N P V As Nb Sb Pr Ta Bi Pa Db Uup 7 15 23 33 41 51 59 73 83 91 105 115 4 O S Cr Se Mo Te Nd W Po U Sg Uuh 8 16 24 34 42 52 60 74 84 92 106 116 5 F Cl Mn Br Tc I Pm Re At Np Bh Uus 9 17 25 35 43 53 61 75 85 93 107 117 6 Ne Ar Fe Kr Ru Xe Sm Os Rn Pu Hs Uuo 10 18 26 36 44 54 62 86 94 108 118 76 7 Co Rh Eu Ir 27 45 63 77 8 Ni Pd Gd Pt Am Mt 95 109 Cm Ds 28 46 64 78 96 9 Cu Ag Tb Au Bk Rg 29 47 65 79 97 111 10 Zn Cd Dv Hg Cf Cn 30 48 66 80 98 112 11 Ho 67 99 12 Er Fm 68 100 13 Tm Md 69 101 14 Electrons in outer shell Yb No 70 102 110 Es Figure 3: The Quantum table ordering elements by the energy levels of their sub shells. Advantages of the Quantum table The Quantum table is simpler and more elegant than the traditional periodic table and other designs. This gives advantages to students studying chemistry as it allows them to understand how the position and properties of an element relate to its atomic structure. The user can visualise the table as an atom with layers of shells. One can see directly by looking at the table, how many electrons are in the outer shell of each element and how many it needs to gain or loose to become stable. For example in the case of a reaction between Oxygen and Hydrogen, it can be seen that hydrogen needs to gain or loose 1 electron and that Oxygen needs to gain two electrons. Since they are both non metals, the simplest structure they could form would have two Hydrogen atoms sharing their outer electrons with a single Oxygen atom, giving the same number of electrons as Neon, with 10 electrons. When lithium reacts with oxygen it forms Li2O or Li2O2. Respectively these have the same number of electrons as Neon (10) and Magnesium (12) which has a full ‘s’ type shell. The table also eases the introduction of students to quantum effects. In terms of visual cohesiveness, the table’s continuous progression includes the Lanthanide and Actinide groups. The table has the same width as the traditional table: 18 elements; and similar depth: 14 elements compared with 9 elements and a gap. Problems with the traditional table Figure 4: Sub shells represented in each row of the traditional periodic table. The left column of figure 4 indicates the sub shells that are filled as the atomic number increases. More than one electron shell is represented in each row. By merging the sub shells together the traditional periodic table reduces the clarity of the data it presents. In addition the 4f and 5f sub shells occur in two different rows, as do the 5d and 6d sub shells, adding further complexity. Use in identifying ‘electron promotion’ Elements such as Copper, Silver and Gold have an unusual arrangements of electrons, thus Silver has 10 electrons in its outer shell rather than 9. This is because an electron that would normally be in a lower sub shell is found instead in the outer sub shell. The Quantum table shown in figure 5 identifies elements with electron promotion and shows the types of electron promotion so that the user is aware of the atomic structure of these elements. Other versions of the periodic table that include sub shells Hundreds of other types of periodic table have been published or have reached the popular media, however these are all variations on a small number of themes. Most relevant to this article is the left step periodic table, the first version of which was first published by Janet in 1928 and more recent versions of which have been popularised by Valery Tsimmerman. This table orders the elements by sub shell, yet groups the sub shells together as in the traditional periodic table, eliminating the benefit. These tables also tend to be very long and thin, making them difficult to follow. Secondly there are many tables designed as spirals and circles to mimic the shape of atoms, one for example, is popularised by Eric Sterri. However these are difficult to use since the atoms are not aligned in order of their atomic number. Use of the table Any predictions that can be made about the properties of elements by their position on the traditional table can be made with the quantum table. Periodicity with regards to atomic radii, ionisation energy, electron affinity, metallic character, electronegativity and atomic emissions all hold true. For example Noble gasses all have full p type outer sub shells. Magnetic materials are all have a 3d type outer sub shell; highly reactive chemicals such as Lithium, Sodium, Potassium and Caesium all have ‘s’ type outer sub shells with 1 electron, i.e. in they are the same row. Calcium, Strontium and Barium all have ‘s’ type outer shells with 2 electrons; Sulphur, Selenium and Tellurium all have ‘p’ type outer shells with 4 electrons; Chlorine, Bromine and Iodine all have ‘p’ type outer shells with 5 electrons; Copper, Silver and Gold all have ‘d’ type outer sub shells with 9 electrons. The Noble metals all have ‘d’ type outer shells with between 6 and 9 electrons in the outer sub shell. Catalysts with similar properties such as Nickel, Palladium and Platinum also all have ‘d’ type outer sub shells but with 8 electrons. For elements with ‘p’ type outer sub shells, the properties change for larger atoms. For example semiconductors all have ‘p’ type outer shells but the number of electrons in the outer shell is higher for higher level p shells. The relative conductivity gradually increases with larger atoms, even with the same number of electrons in the ‘p’ type outer shell. In general, to find an element with a similar property to another one, it is best to examine elements with an outer shell of the same type and a similar number of electrons in the outer shell. Electron Promotion As discussed above, electrons do not always fill the electron shells exactly as would be expected. In some elements a gap appears in an inner shell, and the electron that would normally fill this space is found in the outer shell. There are 2 categories of electron promotion; firstly an electron can jump from a lower shell into the outer shell of this element; this occurs for the elements: Cr, Cu, Nb, Mo, Ru, Rh, Ag, Pt, Pd, Au, Uun and Uub. In the case of Pd, this happens to 2 electrons. In the second category of electron promotion an electron can jump from the outer shell of this element into a higher shell; this occurs in the cases of Gd, Ac, Th, Pa, U, Np and Cm. Details of Electron promotion Examining ‘electron promotion’ in greater detail, one can see that there are four different types of electron promotion: an electron promoted from an ‘s’ type sub shell; two electrons promoted from an ‘s’ type sub shell; an electron promoted from an ‘s’ type sub shell that jumps over an ‘f’ type shell into the outer d shell and an electron promoted from an ‘f’ type sub shell: Promoted 's': an electron from the highest 's' sub shell jumps up to the outer sub shell: Cr, Cu have [Ar] 4s1 3d(N+1) and Nb, Mo, Ru, Rh and Ag have [Kr] 5s1 4d(N+1) Double promoted 's': 2 electrons from the highest 's' sub shell jump up to the outer sub shell: Pd has [Kr] 5s0 4d(N+2) Jumping promoted 's': an electron from the highest 's' sub shell jump up to the outer sub shell and jumps over an 'f' shell to get there: Pt and Au have [Xe] 6s1 4f14 5d(N+1) and Uun and Uub have [Rn] 7s1 5f14 6d(N+1) Promoted 'f': electron from outer 'f' sub shell jumps up to outer sub shell: Gd has [Ba] 4f(N-1) 5d1 and Ac, Th, Pa, U, Np and Cm have [Ra] 5f(N-1) 6d. Where 'N' is the number of electrons in the outer shell. Acknowledgement. The author would like to acknowledge helpful discussions with Rajeeve Dattani and encouragement from Professor Jeremy Ramsden. Figure 5: The Quantum table of the elements, colour version C 12.01 Ar 39.95 ARGON 18 NEON 20.18 Ne 10 Cl 35.45 CHLORINE 17 F 19.00 FLUORINE 9 S 32.07 SULPHUR 16 O 16.00 OXYGEN 8 PHOSPHORUS 30.97 NITROGEN 15 P 14.01 Si 28.09 SILICON 14 Mg 24.31) MAGNESIUM 12 Al ALUMINIUM SODIUM 13 26.98 3p Na 11 22.99 3s N 7 CALCIUM 5 BORON B 10.81 Element Name Atomic Number Symbol Synthetic Relative atomic mass Promoted ‘f’ Gd has [Ba] 4f(N-1) 5d1 Ac, Th, Pa, U, Np & Cm have [Ra] 5f(N-1) 6d1 Jumping promoted ‘s’ Pt & Au have [Xe] 6s1 4f14 5d(N+1) Rg & Cn have [Rn] 7s1 5f14 6d(N+1) Double promoted ‘s’ Pd has [Kr] 5s0 4d(N+2) 40.08 Ca 20 POTASSIUM K 39.10 4s 19 Promoted ‘s’ Cr, Cu have [Ar] 4s1 3d(N+1) Nb, Mo, Ru, Rh & Ag have [Kr] 5s1 4d(N+1) Fe Naturally Occurring f S S S S B BORON CARBON 6 5 10.81 2p Elements with electron promotion Be 9.01 BERYLLIUM 4 HELIUM 4.00 He 2 Li LITHIUM H HYDROGEN 3 6.94 2s Electrons in outer shell 14 13 12 11 10 9 8 7 6 5 4 3 2 1 1 1.01 1s 47.87 50.94 54.94 55.85 87.62 74.92 78.96 79.90 83.80 91.22 Mo 127.6 45 S 102.91 RUTHENIUM S 101.07 Ru 44 TECHNETIUM 43 121.76 127.60 Te 52 ANTIMONY Sb 51 TIN I 126.90 131.29 Actinide Lanthanide 137.33 Not yet discovered BARIUM Ba 56 CAESIUM Cs 132.91 6s 55 Nobel Gases Non-metals Semimetals Alkali metals and Alkali earth metals Transition metals Poor metals XENON Xe 54 IODINE 53 Hydrogen Cd CADMIUM 112.41 SILVER 48 ZINC 65.4 S 107.87 Ag 47 Zn 30 COPPER Cu S 63.55 PALLADIUM 29 NICKEL S S 106.42 Pd 46 Ni 58.69 RHODIUM 28 116.71 Sn 50 INDIUM In 49 114.82 5p MOYBDENIUM TELLURIUM S 95.94 NIOBIUM 42 S 92.91 Nb 41 ZIRCONIUM Zr 40 YITRIUM Y 39 88.91 4d COBALT KRYPTON Kr 36 BROMINE Br 35 SELENIUM Se 34 ARSENIC As 33 GERMANIUM STRONTIUM Sr 38 RUBIDIUM Rb 37 85.47 5s Rh 58.93 72.64 Ge 32 GALLIUM Ga 31 69.72 4p Co 27 IRON Fe 26 MANGANESE Mn 25 CHROMIUM S 52.00) Cr 24 VANADIUM V 23 TITANIUM Ti 22 SCANDIUM Sc 21 44.96 3d 140.91 180.95 Ta 73 144.24 (145) 150.36 183.84 186.21 76 190.23 RHENIUM Re 75 TUNGSTEN W 74 207.20 208.96 (209) (210) 86 (222) ASTATINE At 85 POLONIUM Po 84 BISMUTH Bi 83 LEAD Pb 82 THALLIUM Ti 81 204.38 6p 151.96 158.93 162.50 164.93 167.26 168.93 173.04 YITERBIUM Yb 70 THULIUM Tm 69 ERBIUM Er 68 HOLMIUM Ho 67 DYSPROSIUM Dv 66 TERBIUM Tb 65 GADOLINIUM f 157.25 Gd 64 EUROPIUM Eu 63 SAMARIUM 192.22 MERCURY Hg 200.59 GOLD 80 S 196.97 Au 79 PLATINUM S 195.08 Pt 78 IRIDIUM Ir 77 OSMIUM RADON Sm Os Rn 62 PROMETHIUM 61 NEODYMIUM Nd 60 PRASEODYMIUM TANTALUM Pr 59 Hf 178.49 HAFNIUM 72 CERIUM 140.12 LUTETIUM Lu 71 174.97 5d Ce 58 LANTHANUM La 57 138.91 4f (226) RADIUM Ra 88 FRANCIUM Fr (223) 7s 87 Quantum Table of the Elements f f f (237) (244) (243) (247) (238) (252) (257) (258) (259) NOBELIUM 102 MENDELEVIUM 101 FERMIUM 100 EINSTEINIUM 99 (293) (261) 114 (298) (262) (266) (299) 116 (302) UNUNPENTIUM 115 (264) (277) (268) S (281) 111 S (272) DARMSTADTIUM 110 MEITNERIUM 109 HASSIUM 108 BOHRIUM 107 112 (285) (310) (314) UNUNOCTIUM 118 UNUNSEPTIUM 117 SEABORGIUM UNUNHEXIUM 106 DUBNIUM 105 RUTH’FORDIUM UNUNQUADIUM 104 CALIFORNIUM COPERNICIUM 98 113 (316) (238) UNBINILIUM 120 UNUNENNIUM 119 7p Shell LAWRENCIUM UNUNTRIUM 103 (262) 6d BERKELIUM ROENTGENIUM 97 f (247) CURIUM 96 AMERICIUM 95 PLUTONIUM 94 NEPTUNIUM 93 URANIUM f 238.03 U 92 PROTACTINIUM Pa 231.04 THORIUM 91 f 232.04 Th 90 ACTINIUM Ac 89 (238) 5f Figure 6: The Quantum table of the elements, black and white version C 12.01 Ar 39.95 ARGON 18 NEON 20.18 Ne 10 Cl 35.45 CHLORINE 17 F 19.00 FLUORINE 9 S 32.07 SULPHUR 16 O 16.00 OXYGEN 8 PHOSPHORUS 30.97 NITROGEN 15 P 14.01 Si 28.09 SILICON 14 Mg 24.31) MAGNESIUM 12 Al ALUMINIUM SODIUM 13 26.98 3p Na 11 22.99 3s N 7 CALCIUM 5 BORON B 10.81 Element Name Atomic Number Symbol Synthetic Relative atomic mass Promoted ‘f’ Gd has [Ba] 4f(N-1) 5d1 Ac, Th, Pa, U, Np & Cm have [Ra] 5f(N-1) 6d1 Jumping promoted ‘s’ Pt & Au have [Xe] 6s1 4f14 5d(N+1) Rg & Cn have [Rn] 7s1 5f14 6d(N+1) Double promoted ‘s’ Pd has [Kr] 5s0 4d(N+2) 40.08 Ca 20 POTASSIUM K 39.10 4s 19 Promoted ‘s’ Cr, Cu have [Ar] 4s1 3d(N+1) Nb, Mo, Ru, Rh & Ag have [Kr] 5s1 4d(N+1) Fe Naturally Occurring f S S S S B BORON CARBON 6 5 10.81 2p Elements with electron promotion Be 9.01 BERYLLIUM 4 HELIUM 4.00 He 2 Li LITHIUM H HYDROGEN 3 6.94 2s Electrons in outer shell 14 13 12 11 10 9 8 7 6 5 4 3 2 1 1 1.01 1s 47.87 50.94 54.94 55.85 87.62 74.92 78.96 79.90 83.80 91.22 Mo 127.6 45 S 102.91 RUTHENIUM S 101.07 Ru 44 TECHNETIUM 43 121.76 127.60 Te 52 ANTIMONY Sb 51 TIN I 126.90 131.29 Actinide Lanthanide 137.33 Not yet discovered BARIUM Ba 56 CAESIUM Cs 132.91 6s 55 Nobel Gases Non-metals Semimetals Alkali metals and Alkali earth metals Transition metals Poor metals XENON Xe 54 IODINE 53 Hydrogen Cd CADMIUM 112.41 SILVER 48 ZINC 65.4 S 107.87 Ag 47 Zn 30 COPPER Cu S 63.55 PALLADIUM 29 NICKEL S S 106.42 Pd 46 Ni 58.69 RHODIUM 28 116.71 Sn 50 INDIUM In 49 114.82 5p MOYBDENIUM TELLURIUM S 95.94 NIOBIUM 42 S 92.91 Nb 41 ZIRCONIUM Zr 40 YITRIUM Y 39 88.91 4d COBALT KRYPTON Kr 36 BROMINE Br 35 SELENIUM Se 34 ARSENIC As 33 GERMANIUM STRONTIUM Sr 38 RUBIDIUM Rb 37 85.47 5s Rh 58.93 72.64 Ge 32 GALLIUM Ga 31 69.72 4p Co 27 IRON Fe 26 MANGANESE Mn 25 CHROMIUM S 52.00) Cr 24 VANADIUM V 23 TITANIUM Ti 22 SCANDIUM Sc 21 44.96 3d 140.91 180.95 Ta 73 144.24 (145) 150.36 183.84 186.21 76 190.23 RHENIUM Re 75 TUNGSTEN W 74 151.96 158.93 162.50 164.93 167.26 168.93 173.04 YITERBIUM Yb 70 THULIUM Tm 69 ERBIUM Er 68 HOLMIUM Ho 67 DYSPROSIUM Dv 66 TERBIUM Tb 65 GADOLINIUM f 157.25 Gd 64 EUROPIUM Eu 63 SAMARIUM 192.22 MERCURY Hg 200.59 GOLD 80 S 196.97 Au 79 PLATINUM S 195.08 Pt 78 IRIDIUM Ir 77 OSMIUM Sm Os 62 PROMETHIUM 61 NEODYMIUM Nd 60 PRASEODYMIUM TANTALUM Pr 59 Hf 178.49 HAFNIUM 72 CERIUM 140.12 LUTETIUM Lu 71 174.97 5d Ce 58 LANTHANUM La 57 138.91 4f 207.20 208.96 (209) (210) (222) RADON Rn 86 ASTATINE At 85 POLONIUM Po 84 BISMUTH Bi 83 LEAD Pb 82 THALLIUM Ti 81 204.38 6p (226) RADIUM Ra 88 FRANCIUM Fr (223) 7s 87 Quantum Table of the Elements f f f (237) (244) (243) (247) (238) (252) (257) (258) (259) NOBELIUM 102 MENDELEVIUM 101 FERMIUM 100 EINSTEINIUM 99 (293) (261) 114 (298) (262) (266) (299) 116 (302) UNUNPENTIUM 115 (264) (277) (268) S (281) 111 S (272) DARMSTADTIUM 110 MEITNERIUM 109 HASSIUM 108 BOHRIUM 107 112 (285) (310) (314) UNUNOCTIUM 118 UNUNSEPTIUM 117 SEABORGIUM UNUNHEXIUM 106 DUBNIUM 105 RUTH’FORDIUM UNUNQUADIUM 104 CALIFORNIUM COPERNICIUM 98 113 (316) (238) UNBINILIUM 120 UNUNENNIUM 119 7p Shell LAWRENCIUM UNUNTRIUM 103 (262) 6d BERKELIUM ROENTGENIUM 97 f (247) CURIUM 96 AMERICIUM 95 PLUTONIUM 94 NEPTUNIUM 93 URANIUM f 238.03 U 92 PROTACTINIUM Pa 231.04 THORIUM 91 f 232.04 Th 90 ACTINIUM Ac 89 (238) 5f