NMR investigation of adamantane derivatives of
advertisement

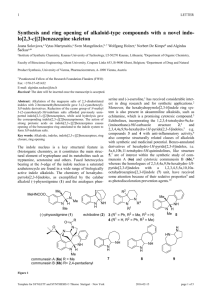
NMR investigation of adamantane derivatives of 1,3-; 1,4-; 2,6- и 2,7-dihydroxynaphthalenes. I.V. Peterson,1 N .M. Svirskaya1, A.A Kondrasenko1 and A.I. Rubaylo1, 2, 3 1 Institute of chemistry and chemical technology SB RAS, 660036, Russia, Krasnoyarsk, Akademgorodok 50, bld.24. 2 Krasnoyarsk scientific centre SB RAS, 660036, Russia, Krasnoyarsk, Akademgorodok 50. 3 Siberian federal university, 660041, Russia, Krasnoyarsk, Svobodniy av. 79. E-mail: Ivan.Peterson.Krsk@gmail.com A great number of the adamantane derivatives have shown many possibilities of practical application[1]. The interaction of dihydroxynaphthalenes with adamantane has not been previously investigated systematically. Thereby, there is no information about the structural features of adamantane derivatives of different dihydroxynaphthalenes. Moreover, recently much attention has been heeded to dearomatization reactions of aromatic compounds due to the highly reactive intermediates opportunities [2]. Current report is our ongoing research of determination structure of adamantyl derivatives of dihydroxynaphthalenes by NMR spectroscopy [3,4] . We describe here assignments and spectral features of 1H and 13C NMR spectra of synthesized 1-adamantyl substituted 1,3- (I); 1,4- (II); 2,6(III) and 2,7- (IV) dihydroxynaphthalenes (Scheme 1). H OH H O OH O 1-AdOH 1-AdOH CF3COOH:CDCl3 in NMR tube I H OH H CH3COOH: H3PO4 H H H O II OH OH 1-AdOH HO III different acid media Ad All NMR spectra (1H, Ad CD2Cl2 inert atmoshere under argon in sealed NMR tube Ad +O2 -H2O Ad= O OH 1-AdOH different acid media IV X Ad OH HO H O Ad morpholine OH HO OH H OH Ad 1-AdOH CF3COOH H CF3COOH:CDCl3 in NMR tube O Ad O OH O H HO OH Ad Ad C, COSY, NOESY, HSQC, HMBC) were collected on a “Bruker 13 Avance III 600” spectrometer system (14.1 T, Krasnoyarsk centre of shared using SB RAS). Reference [1] H. Schwertfeger, A. F. Fokin, P. R. Schreiner. Angew. Chem. Int. Ed., 2008, 47, 1022–1036. [2] M. A. Schätzle, S. Flemming, S. M. Husain, S. Günther, M. Müller. Angew. Chem. Int. Ed., 2012, 51, 2643–2646. [3] I. V. Peterson, N. M. Svirskaya, A. A. Kondrasenko, A. I. Rubaylo. Magn. Reson. Chem., 2013, 51, 762–766. [4] I. V. Peterson, N. M. Svirskaya, A. A. Kondrasenko, A. I. Rubaylo. Magn. Reson. Chem., 2015, 53, in print.