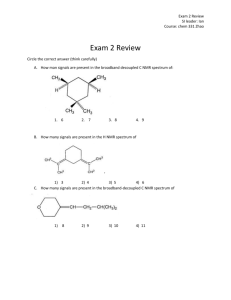
3-benzyl-5-(quinoxalin-3-yl)
advertisement

International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.2, No.4, pp 1987-1991, Oct-Dec 2010 Synthesis of N'-arylidenequinoxaline-2carbohydrazides, 3-Benzyl-5-(quinoxalin-3-yl)1,3,4-oxadiazole-2(3H)-thiones and 1-(2-Aryl5-(quinoxalin-3-yl)-1,3,4-oxadiazol-3(2H)yl)ethanones Thirupathaiah T1, Kranthi kumar T2, Laxminarayana E2 and Thirumala Chary M1* 1Jawaharlal Nehru Technological University College of Engineering, Jagityala,505 327 India 2Sreenidhi Institute of Science and Technology, Ghatkesar, HYDERABAD-501301(A.P.) India *Corres.author:mtcharya@yahoo.com Abstract: Ethyl quinoxaline-2-carboxylate (1), reacts with hydrazine to form Quinoxaline-2-carbohydrazide (2). This compound cyclised with CNBr to offerd 5-(Quinoxalin-3-yl)-1,3,4-oxadiazol-2-amine (3). Compound 3 then converted N'arylidenequinoxaline-2-carbohydrazides (4a-f). The hydrzides (4a-f) are cyclised to form 3-aryl-5-(quinoxalin-3-yl)-1,3,4oxadiazole-2(3H)-thiones (5a-f) and 1-(2-Aryl-5-(quinoxalin-3-yl)-1,3,4-oxadiazol-3(2H)-yl)ethanones (6a-f) by reacting with KOH/CS2 and Ac2O respectively. Keywords: Oxadiazoles, quinoxolines, carbohydrazides, oxadiazathione. Introduction Quinoxaline derivatives are an important class of nitrogencontaining heterocycles in medicinal chemistry13 . For example, quinoxaline is a part of various antibiotics such as echinomycin, levomycin, and actinoleutin that are known to inhibit growth of gram positive bacteria2, and are active against various transplantable tumors3. Several kinds of synthetic routes toward quinoxalines have been developed, inclding the condensation of aryl-1,2-diamines with a 1,2-diketones4, Bi-catalyzed oxidative coupling of epoxides with ene1,2-diamines5, heteroannulation of nitroketene N,Saryliminoacetals with POCl36, cyclization of α-arylimino oximes of α-dicarbonyl compounds7, and formation of α-hydroxy ketones via a tandem oxidation process using Pd(OAc)2 or RuCl2-(PPh3)3-TEMPO8 as well as MnO29. Quinoxaline derivatives have been also reported to possess a wide variety of biological activities10-12. Notable among these are antioxidant, anti-inflammatory antimicrobial, anticancer and antihistamic activities. Drugs having nitrogen containing ring system13-15 are well known for their anti-inflammatory, antioxidant, antihistamic, antimicrobial, antidepressant, hypoglycemic, hypotensive, anticarcenogenic activities etc. 1,3,4-oxadiazoles are also known to have a broad spectrum of biological activities16-18. In addition there are some studies on electronic structures and thiol-thione tautomeric equilibrium of heterocyclic thione derivatives19-21. In view of the above facts, it was contemplated to design and synthesize some oxadiazolo quinoxaline derivatives. M.Thirumala Chary et al /Int.J. ChemTech Res.2010,2(4) 1988 Scheme I N N N CNBr N2H4 O N COOEt N 1 NH2 N CONHNH2 2 3 N N Ar-CHO N Ac2O O N Ar N 6a-e N N KOH CS2 N N 5a-e COCH3 S N Ar 4 a-e O N CONHN N Ar Ar = phenyl, 4-fluorophenyl, 2-fluorophenyl, 2-fluoro-4-methoxyphenyl, 4-hydroxyphenyl, 3-hydroxy-4-methoxyphenyl. Experimental Melting points were measured in a Sulphuric acid bath and are uncorrected. The IR spectra were recorded on Brucher-IFS-66 FTIR instrument. NMR spectra were recorded on Varian Gemini 200 M Hz instrument using tetramethylsilane as an internal standard in CDCl3. Chemical shifts are expressed in ppm. The purity of the compounds was checked by TLC and spots were visualized in iodine vapour. Quinoxaline-2-carbohydrazide (2) To a solution of ethyl quinoxaline-2-carboxylate (1), (0.01 mole) in methanol (15mL) was added 99% hydrazine hydrate (0.05 mole) and refluxed for about 5 hr, reaction was monitored by TLC. After disappearance of starting material reaction mass was cooled to room temperature, and filtered the solid. 1 H NMR in DMSO-d6: 4.72 (brs, 2H), 7.97 (m, 2H), 8.17 (m, 2H), 9.42 (s, 1H), 10.27 (brs, 1H); Mass: m/z 189 (M+1); C9H8N4O; C, 56.44; H, 3.38; N, 30.77; 5-(Quinoxalin-3-yl)-1,3,4-oxadiazol-2-amine (3) To a solution of quinoxaline-2-carbohydrazide, (0.01 mole) in methanol (15mL) was added CNBr (0.01 mole) and refluxed for about 4 hr, reaction was monitored by TLC. After disappearance of starting material reaction mass was cooled to room temperature, and filtered the solid. 1 H NMR in DMSO-d6: 7.73 (brs, 2H), 7.93 (m, 2H), 8.15 (m, 2H), 9.47 (s, 1H); Mass: m/z 214 (M+1); C10H7N5O; C, 57.34; H, 4.32; N, 33.75; N'-arylidenequinoxaline-2-carbohydrazides (4a-f) To a solution of quinoxaline-2-carbohydrazide (0.01 mole) in ethanol (60 ml), aldehyde (0.011 mole) and a few drops of glacial acetic acid were added and the mixture refluxed for 10 hours. It was then cooled concentrated and poured into crushed ice and filtered. The solid thus obtained was purified by recrystallization from ethanol. N'-benzylidenequinoxaline-2-carbohydrazide (4a) 1 H NMR in CDCl3: 7.45 (m, 3H), 7.87 (m, 4H), 8.16 (dd, 1H), 8.22 (dd, 1H), 8.46 (s, 1H), 9.78 (s, 1H), 10.88 (brs, 1H); Mass: m/z 277 (M+1); C16H12N4O; C, 70.25; H, 5.38; N, 21.28; N'-(4-fluorobenzylidene)quinoxaline-2-carbohydrazide (4b) 1 H NMR in CDCl3: 7.45(m, 2H), 7.88 (m, 4H), 8.18 (dd, 1H), 8.23 (dd, 1H), 8.44 (s, 1H), 9.78 (s, 1H), 10.78 (brs, 1H); Mass: m/z 295 (M+1); C16H11FN4O; C, 65.30; H, 3.77; F, 6.46; N, 19.04; O, 5.44 N'-(2-fluorobenzylidene)quinoxaline-2-carbohydrazide (4c) 1 H NMR in CDCl3: 7.43 (m, 2H), 7.82 (m, 4H), 8.12 (dd, 1H), 8.22 (dd,1H), 8.42 (s, 1H), 9.76 (s, 1H), 10.80 M.Thirumala Chary et al /Int.J. ChemTech Res.2010,2(4) (brs, 1H); Mass: m/z 295 (M+1); C16H11FN4O; C, 65.30; H, 3.77; F, 6.46; N, 19.04; N'-(2-fluoro-4-methoxybenzylidene)quinoxaline-2carbohydrazide (4d) 1 H NMR in CDCl3: 3.74 (s, 3H), 7.44 (m, 2H), 7.81 (m, 3H), 8.13 (dd, 1H), 8.22 (dd, 1H), 8.43 (s, 1H), 9.80 (s, 1H), 10.82 (brs, 1H); Mass: m/z 325 (M+1); C17H13FN4O2; C, 63.56; H, 3.44; N, 16.28 N'-(4-hydroxybenzylidene)quinoxaline-2-carbohydrazide (4e) 1 H NMR in CDCl3: 7.35 (m, 3H), 7.81 (m, 3H), 8.13 (dd, 1H), 8.25 (dd, 1H), 8.45 (dd, 1H), 8.25 (dd, 1H), 8.45 (s, 1H), 9.78 (s, 1H), 10.85 (brs, 2H); Mass: m/z 293 (M+1); C16H12N4O2; C, 66.75; H, 5.24; N, 18.17 N'-(3-hydroxy-4-methoxybenzylidene)quinoxaline-2carbohydrazide (4f) 1 H NMR in CDCl3: 3.80(s, 3H), 7.38 (m, 3H), 7.80 (m, 3H), 8.15 (dd, 1H), 8.24 (dd, 1H), 8.43 (s, 1H), 9.75 (s, 1H), 10.80 (brs, 2H); C17H14N4O3; C, 64.55; H, 5.81; N, 16.38 3-Aryl-5-(quinoxalin-3-yl)-1,3,4-oxadiazole-2(3H)thiones (5a-f) To a solution of N'-benzylidenequinoxaline-2carbohydrazide (0.01 mole) in EtOH, KOH (0.01 mole) and CS2 were added and refluxed for overnight. It was then cooled concentrated and poured into crushed ice and filtered. The solid thus obtained was purified by recrystallization from ethanol. 3-benzyl-5-(quinoxalin-3-yl)-1,3,4-oxadiazole-2(3H)thione (5a) 1 H NMR in DMSO-d6: 5.62(s, 2H), 7.25 (m, 5H), 7.85 (m, 2H), 8.22 (dd, 1H), 9.80 (s, 1H); Mass: m/z 321 (M+1) 3-(4-fluorobenzyl)-5-(quinoxalin-3-yl)-1,3,4-oxadiazole2(3H)-thione (5b) 1 H NMR in DMSO-d6: 5.64 (s, 2H), 7.26 (m, 4H), 7.85 (m, 2H), 8.24 (dd, 1H), 9.82 (s, 1H); Mass: m/z 339 (M+1) 3-(2-fluorobenzyl)-5-(quinoxalin-3-yl)-1,3,4-oxadiazole2(3H)-thione (5c) 1 H NMR in DMSO-d6: 5.58 (s, 2H), 7.24 (m, 4H), 7.80 (m, 2H), 8.23 (dd, 1H), 9.82 (s, 1H); Mass: m/z 339 (M+1) 3-(2-fluoro-4-methoxybenzyl)-5-(quinoxalin-3-yl)-1,3,4oxadiazole-2(3H)-thione (5d) 1 H NMR in DMSO-d6: 3.82 (s, 3H), 5.61 (s, 2H), 7.30 (m, 3H), 7.85 (m, 2H), 8.24 (dd, 1H), 9.80 (s, 1H); Mass: m/z 369 (M+1) 1989 3-(4-hydroxybenzyl)-5-(quinoxalin-3-yl)-1,3,4oxadiazole-2(3H)-thione (5e) 1 H NMR in DMSO-d6: 5.60 (s, 2H), 7.28 (m, 4H), 7.80 (m, 2H), 8.25 (dd, 1H), 9.02 (brs, 1H), 9.80 (s, 1H); Mass: m/z 337 (M+1) 3-(3-hydroxy-4-methoxybenzyl)-5-(quinoxalin-3-yl)1,3,4-oxadiazole-2(3H)-thione (5f) 1 H NMR in DMSO-d6: 3.81 (s, 3H), 5.62 (s, 2H), 7.29 (m, 3H), 7.79 (m, 2H), 8.22 (dd, 1H), 9.13 (brs, 1H), 9.82 (s, 1H); Mass: m/z 367 (M+1) 1-(2-Aryl-5-(quinoxalin-3-yl)-1,3,4-oxadiazol-3(2H)yl)ethanones (6a-f) The solution of N'-benzylidenequinoxaline-2carbohydrazide (0.01 mole) and Acetic anhydride (0.01 mole) were refluxed for overnight. It was then cooled concentrated and poured into crushed ice and filtered. The solid thus obtained was purified by recrystallization from ethanol. 1-(2-Phenyl-5-(quinoxalin-3-yl)-1,3,4-oxadiazol-3(2H)yl)ethanones (6a) 1 H NMR in CDCl3: 2.41 (s, 3H), 6.62 (s, 1H), 7.23 (m, 2H), 7.42 (m, 2H), 7.52 (m, 1H), 7.85 (m, 2H), 8.22 (m, 2H), 9.45 (s, 1H); Mass: m/z 319 (M+1) 1-(2-(4-fluorophenyl)-5-(quinoxalin-3-yl)-1,3,4oxadiazol-3(2H)-yl)ethanones (6b) 1 H NMR in CDCl3: 2.40 (s, 3H), 6.60 (s, 1H), 7.24 (m, 2H), 7.43 (m, 2H), 7.65 (m, 2H), 8.22 (m, 2H), 8.45 (s, 1H); Mass: m/z 337 (M+1) 1-(2-(2-fluorophenyl)-5-(quinoxalin-3-yl)-1,3,4oxadiazol-3(2H)-yl)ethanones (6c) 1 H NMR in CDCl3: 2.42 (s, 3H), 6.61 (s, 1H), 7.25 (m, 2H), 7.44 (m, 2H), 7.64 (m, 2H), 8.23 (m, 2H), 9.40 (s, 1H); Mass: m/z 337 (M+1) 1-(2-(2-fluoro-4-methoxyphenyl)-5-(quinoxalin-3-yl)1,3,4-oxadiazol-3(2H)-yl)ethanones (6d) 1 H NMR in CDCl3: 2.39 (s, 3H), 3.78 (s, 3H), 6.60 (s, 1H), 7.13 (m, 2H), 7.44 (m, 2H), 7.65 (m, 2H), 8.22 (m, 1H), 9.39 (s, 1H); Mass: m/z 367 (M+1) 1-(2-(4-hydroxyphenyl)-5-(quinoxalin-3-yl)-1,3,4oxadiazol-3(2H)-yl)ethanones (6e) 1 H NMR in CDCl3: 2.40 (s, 3H), 6.61 (s, 1H), 7.12 (m, 2H), 7.45 (m, 2H), 7.65 (m, 3H), 8.25 (m, 2H), 9.42 (s, 1H); Mass: m/z 335 (M+1) 1-(2-(3-hydroxy-4-methoxyphenyl)-5-(quinoxalin-3-yl)1,3,4-oxadiazol-3(2H)-yl)ethanones (6f) 1 H NMR in CDCl3: 2.40 (s, 3H), 3.86 (s, 3H), 6.59 (s, 1H), 7.13 (m, 2H), 7.44 (m, 2H), 7.66 (m, 2H), 8.26 (m, 2H), 9.45 (s, 1H); Mass: m/z 365 (M+1) M.Thirumala Chary et al /Int.J. ChemTech Res.2010,2(4) 1990 Table 1: Characterization data of compounds (5a-e), (7a-e) and (9a-e) Compd R* Yield m.p. Mol. (%) (oC) formula 5a phenyl 69 222 C17H12N4OS 5b 4-fluorophenyl 71 222 C17H11FN4OS 5c 2-fluorophenyl 73 203 C17H11FN4OS 5d 2-fluoro-4-methoxyphenyl 78 210 C18H13FN4O2S 5e 4-hydroxyphenyl 79 245 C17H12N4O2S 5f 3-hydroxy-4-methoxyphenyl 71 260 C18H14N4O3S 6a phenyl 68 225 C18H14N4O2 6b 4-fluorophenyl 81 232 C18H13FN4O2 6c 2-fluorophenyl 72 250 C18H13FN4O2 6d 2-fluoro-4-methoxyphenyl 75 222 C19H15FN4O3 6e 4-hydroxyphenyl 75 278 C18H14N4O3 6f 3-hydroxy-4-methoxyphenyl 68 242 C19H16N4O4 Conclusions We have developed an efficient protocol synthesis of new 3-benzyl-5-(quinoxalin-3-yl)-1,3,4-oxadiazole2(3H)-thiones (5a-f) and 1-(2-Aryl-5-(quinoxalin-3-yl)1,3,4-oxadiazol-3(2H)-yl)ethanones (6a-f). Operational simplicity, cleaner reaction, easer work-up and are environmentally co-friendly reactions. Moreover quinoxaline derivatives are used as pharmaceutical drugs. References 1. Jaso A, Zarranz B, Aldana I and A. Monge, J. Med. Chem. 2005, 48, 2019; b) Carta A, Paglietti G,. Nikookar M.E.R, Sanna P, Sechi L and Zanetti S, Eur. J. Med. Chem. 2002, 37, 355. 2. Dell, D.H. William, H.R. Morris, G.A. Smith, J.Feeney, G.C.K. Roberts, J. Am. Chem. Soc. 1975, 97, 2497. 3. Bailly C, Echepare S, Gago F and Waring M.J, AntiCancer Drug Des. 1999, 15, 291. 4. a) Heravi M.M, Bakhtiari K, Tehrani M.H, N.M. Javadi and H.A. Oskooie, ARKIVOC 2006, xvi, 16; 5. 6. 7. 8. 9. b) H.R. Darabi, S. Mohandessi, K. Aghapoor and F. Mohsenzadeh, Catal. Commun. 2007, 389; Antoniotti S and Donach E, Tetrahedron Lett. 2002, 43, 3971. Venkatesh C, Singh B, Mahata P.K and Junjapp H, Org. Lett. 7 (2005) 2169. Xekoukoulotakis N P, Hadjiantonious M C P and Maroulis A J, Tetrahedron Lett. 2000, 41, 10299. Robinson R S and Taylor R J K, Synlett (2005) 1003. a) Raw S A Wilfred C D and Taylor R J K, Org. Biomol. Chem, 2004, 2 788; b) Raw S A Wilfred C D and Taylor R J K, Chem. Commun. 2003, 2286. M.Thirumala Chary et al /Int.J. ChemTech Res.2010,2(4) 10. Sandeep K and Devender shinda B, Bioorg Med Chem Lett., 2006, 16, 6181. 11. Dubey P K, Naidu A, Vijaya S and George Vineel B, Indian J Chem., 2005, 44B, 573. 12. Ganapathy S, Ramalingam P and Babu Rao CH, Indian J Heterocycl Chem., 2007, 16, 283. 13. Kumar A, Sharma S and Bajaj K, Indian J Chem., 2003, 42B (8), 1979. 14. Ragabasawaraj B and Sangapure S S, Indian J Heterocycl Chem., 2001, 11, 31. 15. Heas V, Roelof G and Cornelis A, Europ Pat Appl., 1981, 21, 506. ***** 1991 16. Ram,V J and Vlietinck, A J, J Heterocycl. Chem. 1988, 25, 253. 17. Boschelli D H, Connor D T, Bornemeier D A, Dyer R D, Kennedy J A, Kuipers P J, Okonkwo G C, Svhrier D J and Wright C D J. Med. Chem. 1993, 36,1802. 18. Bahadur S and Pandey K K, J. Ind. Chem. Soc. 1980, 57, 447. 19. Aydogan F, Turgut Z Olcay N and Erdem S S, Turk. J. Chem. 2002, 26, 159. 20. Charistos D A, Vagenes G V, Tzavellas L C, Tsoleridis C A and Rodios N A, J. Heterocycl. Chem. 1994, 31, 1593. 21. Tsoleridi C A, Charistos D A and Vagenes G V , J. Heterocycl. Chem. 34, 1997, 1715.