pola27667-sup-0001-suppinfo01
advertisement
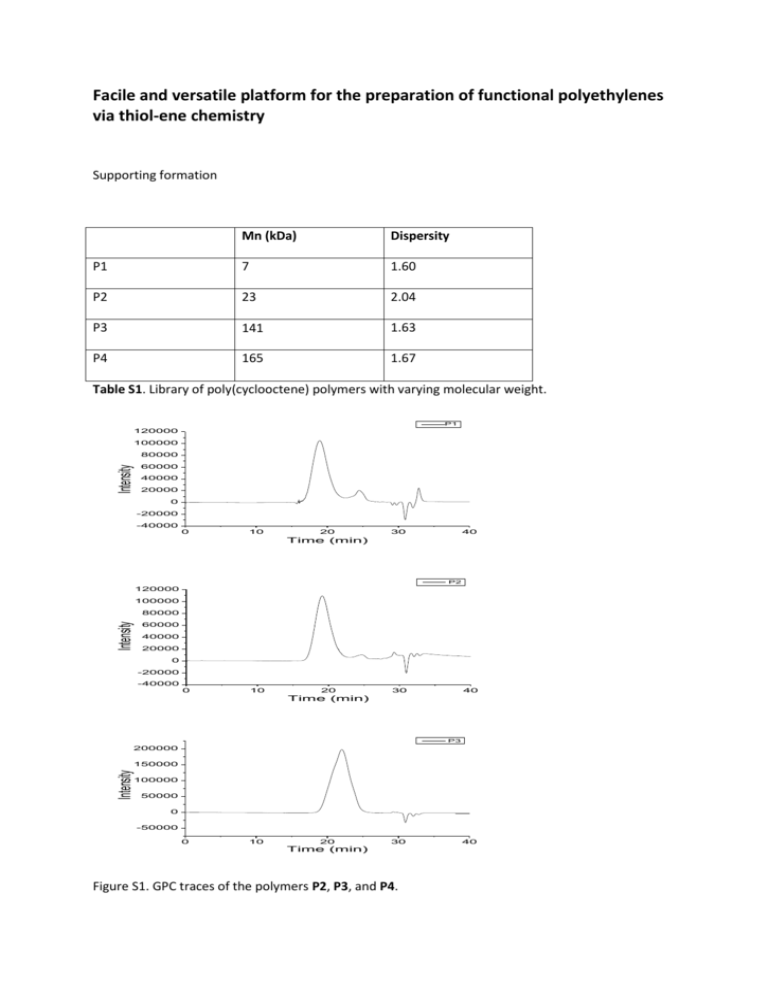
Facile and versatile platform for the preparation of functional polyethylenes via thiol-ene chemistry Supporting formation Mn (kDa) Dispersity P1 7 1.60 P2 23 2.04 P3 141 1.63 P4 165 1.67 Table S1. Library of poly(cyclooctene) polymers with varying molecular weight. P1 120000 100000 Intensity 80000 60000 40000 20000 0 -20000 -40000 0 10 20 30 40 Time (min) P2 120000 100000 Intensity 80000 60000 40000 20000 0 -20000 -40000 0 10 20 30 40 Time (min) P3 200000 Intensity 150000 100000 50000 0 -50000 0 10 20 30 Time (min) Figure S1. GPC traces of the polymers P2, P3, and P4. 40 Figure S2. 1H NMR spectra of the dodecane functional polyethylene (PEdodecane). Figure S3. Conversion of poly(cyclooctene) to 2-mercaptoethanol functional polyethylene followed by 1H NMR. The proton signal of double bond gradually disappears with irradiation. The conversion rate at 0.5h, 1h, 2h, 4h, and 6 h, are 38%, 69%, 81%, 88%, 93%, respectively (calculated from the integration ratio of the double bond and the methylene group of mercaptoethanol. Figure S4. 1H NMR spectrum of the thioglycolic acid functional polyethylene (PEthioglycolic acid) in d6DMSO.











