Warm-up:
advertisement

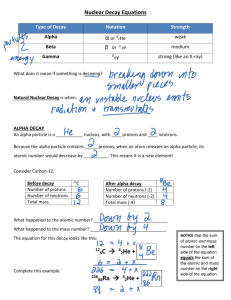
TEACHER NOTES (2) – Nuclear Chemistry 12.A TOPIC 3 - TYPES OF RADIOACTIVE DECAY 1. Education Portal (tutorial) : Alpha, Beta, Gamma types of radiation 2. Reading Activity – Types of Radioactive Decay + Questions (sent to you earlier) 3. Notes (make your own ) Nuclear Reactions Chemical reactions involve the breaking and forming of bonds between different atoms. In a nuclear reaction the situation is different – in a nuclear reaction changes occur involving the number of protons, neutrons, or electrons in a single atom. Proton is symbolized 1 Electron 0 0 Neutron: 1 p e OR n 1 -1 -1 0 Radioactive Decay – a spontaneous (natural – doesn’t require energy) process in which unstable nuclei lose energy by emitting radiation (alpha, beta, gamma) to increase stability. Atoms of one element can change into atoms of another element. Atoms with mass numbers around 60 are the most stable. Why do radioactive atoms (radioisotopes) emit radiation? Because their nuclei are unstable (has to do with the neutron – proton ratio. Ex. C-12 is stable neutron to proton ration is 1:1; C-14 is unstable neutron to proton ratio is 1.33 : 1. too many neutrons causes a nucleus to be unstable). How do these atoms gain stability? By losing energy through radioactive decay. Types of Radioactive Decay A. Alpha Emission – an alpha particle () is 2 protons and 2 neutrons (or a helium nucleus) bound together and is emitted from the unstable nucleus during radioactive decay. α particle = Helium nucleus = High energy = heavy = low speed =(2+) charged particles= 42 He (helium-4) = 42α Example 1: 210 206 Po Pb 84 4 He 2 + 82 polonium – 210 lead – 206 alpha particle Example 2: 238 92 U→ Uranium – 238 234 90 Th + Thorium – 234 4 2 He alpha particle Clothes/skin/paper will shield you form alpha particles. B. Beta Emission – a beta particle () is an electron emitted from the unstable nucleus when a neutron is converted to a proton. n → 11 p + 0−1e β particle = electron = high energy = light = high speed =(1-) charged electrons = 1 0 0 -1e = 0-1β Examples: 14 C 6 carbon – 14 14 N + 7 nitrogen – 14 0 e -1 beta particle Metal foil will shield you from beta particles. C. Gamma Emission or gamma rays ( ) are high-energy electromagnetic waves emitted from a nucleus as it changes from an excited state to a ground energy state. Very similar to light, but is much more dangerous. Gamma emission usually occurs immediately following other types of decay (alpha and beta) and account for most of the energy lost during the radioactive decay process. γ “particle” (EM energy) = (short wavelength/high frequency) high energy photons = NO mass charge = very penetrating (harmful) = 00γ = NO Example: 238 92 U→ Uranium – 238 234 90 Th + Thorium – 234 4 2 He + 2 00 (two gamma rays of two different frequencies) alpha particle gamma rays Lead or concrete will protect you from gamma rays. Summary: Property Alpha(α) Beta (β) Composition Alpha particles Beta particles What is it? (Description of radiation) Helium nuclei 4 4 2 He or 2α Electrons 0 0 -1e or -1β Charge Speed Energy positive 2+ 6.64 x 10-24 kg (heavy) Decreases the mass number by 4 Decreases the atomic number by 2 Low (slow moving) Low negative 19.11 x 10-28 kg (light) Converts a neutron into proton and electron Increases atomic number by 1 High (fast moving) High Relative penetrating power Blocked by paper or clothing Blocked by metal foil Mass How it changes the nucleus Gamma () High-energy electromagnetic radiation Photons 0 0 neutral 0 (no charge) 0 (no mass) No change in mass number nor atomic number Highest speed Highest (most penetrating) Not completely blocked by lead or concrete 12.B TOPIC 4 – Balancing Nuclear Equations Need to remember: In balanced nuclear equations, the sum of the mass numbers (superscripts) is balanced (equal) on both sides of the equation. In balanced nuclear equations, the sum of the atomic numbers (subscripts) is balanced (equal) on both sides of the equation. Example – Alpha Decay: Answer: 228 88 Example – Beta Decay: X + 0=60; x = 60 Answer: 60 27Co x y? + 0 -1e Ra 60 28 Ni Y + (-1) = 27; y = 27+1 = 28 Characteristics of Chemical and Nuclear Reactions Chemical reactions Nuclear reactions 1. Occur when bonds are broken and formed 1. Occur when nuclei emit particles and/or rays 2. Atoms unchanged, rearranged 2. Atoms converted into atoms of another element 3. Involve only valence electrons 3. Involve p+, e-, n0 4. Release/absorb small amounts of energy 4. Release/absorb LARGE amount of energy 5. Reaction rate is NOT influenced by T’, P, concentration, 5. Reaction rate is influenced by T’, P, concentration, catalysts catalysts