chemistryhelprush.wikispaces.com
advertisement

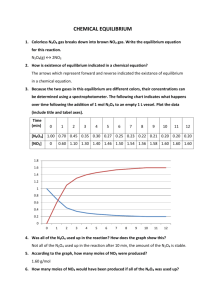
AP Chemistry Practice Test Zumdahl Chapter 12 Kinetics and 13 Equilibrium This is converted using RTF so some of the symbols are strange. Infinity is really times. Looks like has been replaced by an O with a slash. You should be able to figure out most of them. Sorry, but I don’t have time to do more. 1. The average rate of disappearance of ozone in the reaction is found to be 8.49 10–3 atm over a certain interval of time. What is the rate of appearance of O2 during this interval? A) 12.7 10–3 atm/s B) 8.49 10–3atm/s C) 5.66 10–3atm/s D) 306 10–3atm/s E) 24.0 10–3atm/s 2. The balanced equation for the reaction of bromate ion with bromide ion in acidic solution is given by: At a particular instant in time, the value of –[Br–]/t is 2.0 10–3 mol/L s. What is the value of [Br2]/t in the same units? A) 1.2 10–3 B) 2.0 10–3 C) 3.3 10–3 D) 1.0 10–3 E) 1.7 10–3 3. Consider the reaction 2H2 + O2 2H2O What is the ratio of the initial rate of the appearance of water to the initial rate of disappearance of oxygen? A) 1 : 1 B) 2 : 1 C) 1 : 2 D) 2 : 2 E) 3 : 2 4. Consider the reaction: 4NH3 + 7O2 4NO2 + 6H2O At a certain instant the initial rate of disappearance of the oxygen gas is X. What is the value of the appearance of water at the same instant? A) 1.2 X B) 1.1 X C) 0.86 X D) 0.58 X E) cannot be determined from the data 5. For the reaction 5A + 3B ? 5C + 4D, at a particular instant in time, the rate of the reaction is 0.0272 M/s. What is the rate of change of A? A) –0.0272 M/s B) 0.136 M/s C) –0.136 M/s D) –0.00544 M/s E) 0.00544 M/s 6. Consider the reaction X Y + Z Which of the following is a possible rate law? A) Rate = k[X] B) Rate = k[Y] C) Rate = k[Y][Z] D) Rate = k[X][Y] E) Rate = k[Z] 7. Consider the following rate law: Rate = k[A]n[B]m How are the exponents n and m determined? A) by using the balanced chemical equation B) by using the subscripts for the chemical formulas C) by using the coefficients of the chemical formulas D) by educated guess E) by experiment 8. The following data were obtained for the reaction of NO with O2. Concentrations are in molecules/cm3 and rates are in molecules/cm3s. [NO]0 [O2]0 Initial Rate 1 ¥ 1018 1 ¥ 1018 2.0 ¥ 1016 18 18 2 ¥ 10 1 ¥ 10 8.0 ¥ 1016 3 ¥ 1018 1 ¥ 1018 18.0 ¥ 1016 18 18 1 ¥ 10 2 ¥ 10 4.0 ¥ 1016 1 ¥ 1018 3 ¥ 1018 6.0 ¥ 1016 What is the rate law? A) Rate = k[NO][O2] B) Rate = k[NO][O2]2 C) Rate = k[NO]2[O2] D) Rate = k[NO]2 E) Rate = k[NO]2[O2]2 9. The reaction of (CH3)3CBr with hydroxide ion proceeds with the formation of (CH3)3COH. (CH3)3CBr(aq) + OH–(aq) (CH3)3COH(aq) + Br–(aq) The following data were obtained at 55C. [(CH3)3CBr]0 [OH–]0 Initial Rate Exp. (mol/L) (mol/L) (mol/L) 1 0.10 0.10 1.0 ¥ 10–3 2 0.20 0.10 2.0 ¥ 10–3 3 0.10 0.20 1.0 ¥ 10–3 4 0.30 0.20 ? What will the initial rate (in mol/L·s) be in Experiment 4? A) 3.0 10–3 B) 6.0 10–3 C) 9.0 10–3 D) 18 10–3 E) none of these 10. For a reaction in which A and B react to form C, the following initial rate data were obtained: [A] [B] Initial Rate of Formation of C (mol/L·s) 1.00 4.00 8.00 (mol/L) (mol/L) 0.10 0.10 0.10 0.20 0.20 0.20 What is the rate law? A) Rate = k[A][B] B) Rate = k[A]2[B] C) Rate = k[A][B]2 D) Rate = k[A]2[B]2 E) Rate = k[A]3 11. Tabulated below are initial rate data for the reaction 2Fe(CN)63– + 2I– 2Fe(CN)64– + I2 Run [Fe(CN)63–]0 1 0.01 2 0.01 3 0.02 4 0.02 5 0.02 The experimental rate law is: [I–]0 0.01 0.02 0.02 0.02 0.02 [Fe(CN)64–]0 0.01 0.01 0.01 0.02 0.02 [I2]0 0.01 0.01 0.01 0.01 0.02 Initial Rate (M/s) 1 ¥ 10–5 2 ¥ 10–5 8 ¥ 10–5 8 ¥ 10–5 8 ¥ 10–5 A) = k[Fe(CN)63–]2[I–]2[Fe(CN)64–]2[I2] B) = k[Fe(CN)63–]2[I–][Fe(CN)64–][I2] C) = k[Fe(CN)63–)]2[I–] D) = k[Fe(CN)63–][I–]2 E) = k[Fe(CN)63–][I–] [Fe(CN)64–] 12. Tabulated below are initial rate data for the reaction: 2Fe(CN)63– + 2I– 2Fe(CN)64– + I2 Run [Fe(CN)63–]0 1 0.01 2 0.01 3 0.02 4 0.02 5 0.02 What is the value of k? A) 107 M–5 s–1 B) 103 M–3 s–1 C) 10 M–2 s–1 D) 50 M–2 s–1 E) none of these [I–]0 0.01 0.02 0.02 0.02 0.02 [Fe(CN)64–]0 0.01 0.01 0.01 0.02 0.02 [I2]0 0.01 0.01 0.01 0.01 0.02 Rate (M/s) 1 ¥ 10–5 2 ¥ 10–5 8 ¥ 10–5 8 ¥ 10–5 8 ¥ 10–5 Use the following to answer questions 13-18. A general reaction written as A + 2B C + 2D is studied and yields the following data: [A]0 [B]0 Initial D[C]/Dt 0.150 M 0.150 M 8.00 ¥ 10–3 mol/L·s 0.150 M 0.300 M 1.60 ¥ 10–2 mol/L·s 0.300 M 0.150 M 3.20 ¥ 10–2 mol/L·s 13. What is the order of the reaction with respect to B? A) 0 B) 1 C) 2 D) 3 E) 4 14. What is the order of the reaction with respect to A? A) 0 B) 1 C) 2 D) 3 E) 4 15. What is the overall order of the reaction? A) 0 B) 1 C) 2 D) 3 E) 4 16. What is the numerical value of the rate constant? A) 0.053 B) 1.19 C) 2.37 D) 5.63 E) none of these (A-D) 17. Determine the initial rate of B consumption ([B]/t) for the first trial? A) 8.00 10–3 mol/L·s B) 1.60 10–2 mol/L·s C) 3.20 10–2 mol/L·s D) 4.00 10–3 mol/L·s E) none of these (A-D) 18. Determine the initial rate of C production ([C]/t) if [A] = 0.200 M and [B] = 0.500 M. A) 4.74 10–2 mol/L·s B) 2.37 10–1 mol/L·s C) 1.19 10–1 mol/L·s D) 8.23 10–2 mol/L·s E) none of these (A-D) Use the following to answer questions 19-21. Consider the following data concerning the equation: H2O2 + 3I– + 2H+ I3– + 2H2O [H2O2] [I–] I 0.100 M 5.00 10–4M II. 0.100 M 1.00 10–3M III. 0.200 M 1.00 10–3M [H+] 1.00 10–2M 1.00 10–2M 1.00 10–2M rate 0.137 M/sec 0.268 M/sec 0.542 M/sec IV. 0.400 M 1.00 10–3M 2.00 10–2M 1.084 M/sec 19. The rate law for this reaction is A) rate = k[H2O2][I–][H+] B) rate = k[H2O2]2[I–]2[H+]2 C) rate = k[I–][H+] D) rate = k[H2O2][H+] E) rate = k[H2O2][I–] 20. The average value for the rate constant k (without units) is A) 2710 B) 2.74 104 C) 137 D) 108 E) none of these 21. Two mechanisms are proposed: A) Mechanism I, with the first step the rate determining step. B) Mechanism I, with the second step the rate determining step. C) Mechanism II, with the first step rate determining. D) Mechanism II, with the second step rate determining. E) None of the above could be correct. Use the following to answer questions 22-23. The following initial rate data were found for the reaction 2 ¥ 10–3 22. Which of the following is the correct rate law? A) Rate = k[MnO4–]2[H2C2O4]5[H+]6 B) Rate = k[MnO4–]2[H2C2O4][H+] C) Rate = k[MnO4–][H2C2O4][H+] D) Rate = k[MnO4–]2[H2C2O4] 2MnO4– + 5H2C2O4 + 6H+ Æ 2Mn2+ + 10CO2 + 8H [MnO4–]0 [H2C2O4]0 [H+]0 1 ¥ 10–3 1 ¥ 10–3 1.0 –3 2 ¥ 10 1 ¥ 10–3 1.0 –3 –3 2 ¥ 10 2 ¥ 10 1.0 2 ¥ 10–3 2.0 E) Rate = k[MnO4–]2[H2C2O4]2 23. What is the value of the rate constant? A) 2 105 Ms–1 B) 2 105 M–2s–1 C) 200 M–1s–1 D) 200 M–2s–1 E) 2 10–4 Ms–1 Use the following to answer questions 24-27. The following questions refer to the reaction between nitric oxide and hydrogen 2NO + H2 N2O + H2O Experiment Initial [NO], M Initial [H2], M Initial Rate of Disappearance of NO (mol/L sec) 1 6.4 ¥ 10–3 2.2 ¥ 10–3 2.7 ¥ 10–5 2 3 12.8 ¥ 10–3 6.4 ¥ 10–3 2.2 ¥ 10–3 4.5 ¥ 10–3 24. What is the rate law for this reaction? A) Rate = k[NO] B) Rate = k[NO]2 C) Rate = k[NO]2[H2] D) Rate = k[NO][H2] E) Rate = k[N2O][H2O] 25. What is the magnitude of the rate constant for this reaction? A) 0.66 B) 4.2 10–3 C) 870 D) 1.9 E) 300 26. What are the units for the rate constant for this reaction? A) L/mol·s B) L2/mol2·s C) mol/L·s D) s–2 E) L–2 1.1 ¥ 10–4 5.4 ¥ 10–5 27. What is the order of this reaction? A) 3 B) 2 C) 1 D) 0 E) cannot be determined from the data Use the following to answer questions 28-29. The reaction H2SeO3(aq) 6I–(aq) + 4H+(aq) 2I3–(aq) + 3H2O(l) + Se(s) was studied at 0°C by the method of initial rates: [H2SeO3]0 1.0 ¥ 10–4 2.0 ¥ 10–4 3.0 ¥ 10–4 1.0 ¥ 10–4 1.0 ¥ 10–4 1.0 ¥ 10–4 1.0 ¥ 10–4 [H+]0 2.0 ¥ 10–2 2.0 ¥ 10–2 2.0 ¥ 10–2 4.0 ¥ 10–2 1.0 ¥ 10–2 2.0 ¥ 10–2 4.0 ¥ 10–2 [I–]0 2.0 ¥ 10–2 2.0 ¥ 10–2 2.0 ¥ 10–2 2.0 ¥ 10–2 2.0 ¥ 10–2 4.0 ¥ 10–2 4.0 ¥ 10–2 Rate (mol/L s) 1.66 ¥ 10–7 3.33 ¥ 10–7 4.99 ¥ 10–7 6.66 ¥ 10–7 0.41 ¥ 10–7 13.4 ¥ 10–7 5.33 ¥ 10–6 28. The rate law is A) Rate = k[H2SeO3][H+][I–] B) Rate = k[H2SeO3][H+]2[I–] C) Rate = k[H2SeO3][H+][I–]2 D) Rate = k[H2SeO3]2[H+][I–] E) Rate = k[H2SeO3][H+]2[I–]3 29. The numerical value of the rate constant is A) 5.2 105 B) 2.1 102 C) 4.2 D) 1.9 10–6 E) none of these Use the following to answer questions 30-33. The following questions refer to the reaction shown below: Experiment 1 2 3 Initial [A] (mol/L) 0.16 0.16 0.08 Initial [B] (mol/L) 0.15 0.30 0.30 Initial Rate of Disappearance of A (mol/L·s) 0.08 0.30 0.08 30. What is the rate law for this reaction? A) Rate = k[A][B] B) Rate = k[A]2[B] C) Rate = k[A][B]2 D) Rate = k[A]2[B]2 E) Rate = k[B] 31. What is the magnitude of the rate constant for the reaction? A) 140 B) 79 C) 119 D) 164 E) 21 32. What are the units for the rate constant for this reaction? A) L/mol·s B) L2/mol2·s C) mol/L·s D) L3/mol3·s E) mol3/L 33. What is the order of this reaction? A) 4 B) 3 C) 2 D) 1 E) 0 34. Initial rate data have been determined at a certain temperature for the gaseous reaction 2NO + 2H2 N2 + 2H2O. [NO]0 [H2]0 Initial Rate (M/s) 0.10 0.20 0.0150 0.10 0.30 0.0225 0.20 0.20 0.0600 What is the value of the rate constant? A) 7.5 B) 3.0 10–3 C) 380 D) 0.75 E) 3.0 10–4 35. The following data were obtained at 25°C: [A]0 [B]0 [C]0 0.1 0.2 0.3 0.3 0.4 0.2 0.6 0.4 0.2 0.3 0.4 0.1 0.6 0.2 0.2 What is the correct rate law? A) Rate = k[A][B][C] B) Rate = k[A][B][C]2 C) Rate = k[A][C] D) Rate = k[A]3[B]2[C] E) Rate = k[A][C]2 Rate 0.063 0.084 0.168 0.021 0.168 Use the following to answer questions 36-38. The oxidation of Cr3+ to CrO42– can be accomplished using Ce4+ in a buffered solution. The following data were obtained: Relative Initial Rate [Ce4+]0 [Ce3+]0 [Cr3+]0 1 2 4 16 2.0 ¥ 10–3 4.0 ¥ 10–3 4.0 ¥ 10–3 8.0 ¥ 10–3 1.0 ¥ 10–2 2.0 ¥ 10–2 1.0 ¥ 10–2 2.0 ¥ 10–2 36. Determine the order in the rate law of the species Ce4+. A) 1 B) 2 C) 3 D) –1 E) –2 3.0 ¥ 10–2 3.0 ¥ 10–2 3.0 ¥ 10–2 6.0 ¥ 10–2 37. Determine the order in the rate law of the species Ce3+. A) 1 B) 2 C) 3 D) –1 E) –2 38. Determine the order in the rate law of the species Cr3+. A) 1 B) 2 C) 3 D) –1 E) –2 39. The rate expression for a particular reaction is rate = k[A][B]2. If the initial concentration of B is increased from 0.1 M to 0.3 M, the initial rate will increase by which of the following factors? A) 2 B) 6 C) 12 D) 3 E) 9 40. The following data were obtained for the reaction 2A + B C where rate = [C]/t [A](M) [B](M) Initial Rate (M/s) 0.100 0.0500 2.13 ¥ 10–4 0.200 0.0500 1.70 ¥ 10–2 0.400 0.100 1.36 ¥ 10–1 What is the value of the rate constant? A) 2.13 B) 0.213 C) 0.426 D) 1.70 E) none of these 41. The reaction 2A + 5B ? products is third order in A and third order in B. What is the rate law for this reaction? A) rate = k[A]2[B]5 B) rate = k[A]3[B]3 C) rate = k[A]3[B]3 D) rate = k[A]5[B]2 E) rate = k[A]2/7[B]5/7 42. The reaction 3A + 4B ? products is first order in A and third order in B. What is the overall order of the reaction? A) 0 B) 7 C) 1 D) 4 E) 3 43. The reactants A and B are mixed, and the reaction is timed until a color change occurs. The data are as follows: [A] 0.100 0.050 0.100 [B] 0.140 0.140 0.070 Time (s) 25 50 100 The order of the reaction in terms of B is A) 2. B) 1/2. C) 0. D) –1/2. E) 1. 44. The reaction has the following rate law: After a period of 2.4 103 s, the concentration of NO falls from an initial value of 2.8 10–3 mol/L to 2.0 10–3 mol/L. What is the rate constant, k? A) 1.7 10–7 M–1·s–1 B) 6.0 10–2 M–1·s–1 C) 3.6 10–1 M–1·s–1 D) 1.4 10–4 M–1·s–1 E) 3.0 10–2 M–1·s–1 45. The following data were collected for the decay of HO2 radicals: Time [HO2] Time [HO2] 0s 1.0 ¥ 1011 molec/cm3 14 s 1.25 ¥ 1010 molec/cm3 2s 5.0 ¥ 1010 molec/cm3 30 s 6.225 ¥ 109 molec/cm3 10 3 6s 2.5 ¥ 10 molec/cm A) The decay of HO2 occurs by a first-order process. B) The half-life of the reaction is 2 ms. C) A plot of ln [HO2] versus time is linear with a slope of –k. D) The rate of the reaction increases with time. E) A plot of 1/[HO2] versus time gives a straight line. 46. A first-order reaction is 45% complete at the end of 36 minutes. What is the length of the half-life of this reaction? A) 42 min B) 31 min C) 2.2 h D) 52 min E) none of these Use the following to answer questions 47-50. The following questions refer to the gas-phase decomposition of ethylene chloride. C2H5Cl products Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction: Time (s) 1.0 2.0 ln [C2H5Cl] (M) –1.625 –1.735 47. What is the rate constant for this decomposition? A) 0.29/s B) 0.35/s C) 0.11/s D) 0.02/s E) 0.22/s 48. What was the initial concentration of the ethylene chloride? A) 0.29 M B) 0.35 M C) 0.11 M D) 0.02 M E) 0.22 M 49. What would the concentration be after 5.0 seconds? A) 0.13 M B) 0.08 M C) 0.02 M D) 0.19 M E) 0.12 M 50. What is the half-life time for this reaction? A) 0.7 s B) 1.3 s C) 8.9 s D) 6.3 s E) 2.2 s 51. For a reaction: aAProducts, [A]0 = 5.4 M, and the first two half-lives are 56 and 28 minutes, respectively. Calculate k (without units). A) 9.6 10–2 B) 3.3 10–3 C) 4.8 10–2 D) 6.6 10–3 E) none of these 52. For a reaction: aAProducts, [A]0 = 6.0 M, and the first two half-lives are 56 and 28 minutes, respectively. Calculate [A] at t = 105.3 minutes. A) 5.6 M B) 12 M C) 0.71 M D) 0.36 M E) none of these 53. For which order reaction is the half-life of the reaction proportional to 1/k (k is the rate constant)? A) zero order B) first order C) second order D) all of the above E) none of the above Use the following to answer questions 54-56. The kinetics of the reaction A + 3B C + 2D were studied and the following results obtained, where the rate law is: – DSMTEF050101050242524545350000 1357696E416C6C4261736963436F646 5506167657300110554696D6573204E 657720526F6D616E00110353796D62 6F6C0011044D5420457874726100120 008212F458F442F4150F4100F475F41 50F21F1E4150F4150F4100F445F425F 48F425F4100F4100F435F4100F48F45 F42A5F48F48F4100F4100F40F48F41 7F48F4100F412A5F445F45F45F45F4 5F410F0C01000100010202020200020 00101010000000300000A010003000B 00000100020485940344030003030001 000200814100000200965B000200965 D000000010002048594034402008374 A 0000000000 = k[A]n[B]m t For a run where [A]0 = 1.0 10–3 M and [B]0 = 5.0 M, a plot of ln [A] versus t was found to give a straight line with slope = –5.0 10–2 s–1. For a run where [A]0 = 1.0 10–3 M and [B]0 = 10.0 M, a plot of ln [A] versus t was found to give a straight line with slope = –7.1 10–2 s–1. 54. What is the value of n? A) 0 B) 0.5 C) 1 D) 1.5 E) 2 55. What is the value of m? A) 0 B) 0.5 C) 1 D) 1.5 E) 2 56. Calculate the value of k (ignore units). A) 22 B) 10 C) 50 D) 1.1 E) none of these Use the following to answer questions 57-61. For the reaction 2N2O5(g) 4NO2(g) + O2(g), the following data were collected: t (minutes) [N2O5] (mol/L) 0 1.24 ¥ 10–2 10. 0.92 ¥ 10–2 20. 0.68 ¥ 10–2 30. 0.50 ¥ 10–2 40. 0.37 ¥ 10–2 50. 0.28 ¥ 10–2 70. 0.15 ¥ 10–2 57. The order of this reaction in N2O5 is A) 0 B) 1 C) 2 D) 3 E) none of these 58. The concentration of O2 at t = 10. minutes is A) 2.0 10–4 mol/L B) 0.32 10–2 mol/L C) 0.16 10–2 mol/L D) 0.64 10–2 mol/L E) none of these 59. The initial rate of production of NO2 for this reaction is approximately A) 7.4 10–4 mol/L·min B) 3.2 10–4 mol/L·min C) 1.24 10–2 mol/L·min D) 1.6 10–4 mol/L·min E) none of these 60. The half-life of this reaction is approximately A) 15 minutes B) 18 minutes C) 23 minutes D) 36 minutes E) 45 minutes 61. The concentration N2O5 at 100 minutes will be approximately A) 0.03 10–2 mol/L B) 0.06 10–2 mol/L C) 0.10 10–2 mol/L D) 0.01 10–2 mol/L E) none of these Use the following to answer questions 62-64. The following questions refer to the hypothetical reaction A + B products. The kinetics data given can be analyzed to answer the questions. [A]0 [B]0 Rate of decrease (mol/L) (mol/L) of [A] (M/s) 5.0 5.0 X 10.0 5.0 2X 5.0 10.0 2X Time (s) [B] (mol/L) 10.0 100 20.0 100 30.0 100 62. The rate law for the reaction is Rate = k[A]x[B]y. What are the values of x and y? A) x = 0 y = 1 B) x = 1 y = 0 C) x = 1 y = 1 D) x = 2 y = 1 E) x = 1 y = 2 63. What form will the pseudo-rate law have? A) Rate = k'[A]x B) Rate = k'[B]y C) Rate = k'[A]x[B]y D) Rate = kk'[A]x E) Rate = kk'[B]y 64. Determine the magnitude of the pseudo-rate constant (k') if the magnitude of X in the rate data is 0.00905. A) 4.3 10–3 B) 1.2 10–2 C) 0.86 D) 0.31 E) 1.81 10–3 Use the following to answer questions 65-66. The reaction A B + C is known to be zero order in A with a rate constant of 5.0 10– 2 mol/L·s at 25°C. An experiment was run at 25°C where [A]0 = 1.0 10–3 M. 65. The integrated rate law is A) [A] = kt B) [A] – [A]0 = kt C) D) ln E) [A]0 – [A] = kt 66. What is the concentration of B after 5 10–3 sec? A) 5.0 10–5 M B) 5.0 10–4 M C) 7.5 10–4 M D) 2.5 10–4 M E) none of these 67. The reaction is known to be zero order in A with a rate constant of 5.0 10–2 mol/L s at 25°C. An experiment was run at 25°C where [A]0 = 3.0 10–3 M. After 5.0 minutes, the rate is A) 5.0 10–2 mol/L·s B) 2.5 10–2 mol/L·s C) 1.3 10–2 mol/L·s D) 3.0 10–3 mol/L·s E) none of these 68. The reaction is known to be zero order in A with a rate constant of 5.0 10–2 mol/L s at 25°C. An experiment was run at 25°C where [A]0 = 3.0 10–3 M. The halflife for the reaction is A) 3.0 10–2s B) 3.0 102s C) 5.0 10–2 s D) 6.0 10–4s E) none of these 69. The reaction exhibits the rate law where k = 1.0 10–5 M–1 s–1 at 25°C. This reaction is run where the initial concentration of NOBr ([NOBr]0) is 0.15 M. What is one half-life for this experiment? A) 1.5 s B) 0.8 10–5 s C) 6.9 10–4 s D) 6.7 105 s E) none of these 70. The reaction 2NOBr 2NO + Br2 exhibits the rate law Rate = k[NOBr]2 = – DSMTEF0501010502425245453500001357 696E416C6C4261736963436F64655061676 57300110554696D6573204E657720526F6D 616E00110353796D626F6C0011044D5420 457874726100120008212F458F442F4150F4 100F475F4150F21F1E4150F4150F4100F44 5F425F48F425F4100F4100F435F4100F48F 45F42A5F48F48F4100F4100F40F48F417F4 8F4100F412A5F445F45F45F45F45F410F0 C01000100010202020200020001010100000 00300000A010003000B0000010002048594 0344030003030001000200814E000200814F 0002008142000200817200000200965B0002 00965D000000010002048594034402008374 NOBr 0000000000 t where k = 1.0 10–5 M–1 s–1 at 25°C. This reaction is run where the initial concentration of NOBr ([NOBr]0) is 1.00 10–1 M. The [NO] after 1.00 hour has passed is A) 3.6 10–4 M B) 9.9 10–3 M C) 9.7 10–3 M D) 1.0 10–3 M E) none of these Use the following to answer questions 71-72. For the reaction A Products, successive half-lives are observed to be 10.0 min and 40.0 min. 71. The reaction follows the integrated rate law A) [A] = –kt + [A]0 B) ln [A] = –kt + ln [A]0 C) = kt + D) = kt + E) none of these 72. At the beginning of the reaction, [A] was 0.68 M. The numerical value of the rate constant is A) 0.069 B) 0.15 C) 10. D) 0.034 E) none of these 73. The reaction is first order in N2O5. For this reaction at 45oC, the rate constant k = 1.0 10–5 s–1, where the rate law is defined as For a particular experiment ([N2O5]0 = 1.0 10–3 M), calculate [N2O5] after 2.7 105 seconds. A) 2.7 M B) 1.0 10–3 M C) 6.7 10–5 M D) 0 M E) 9.6 M Use the following to answer questions 74-77. Consider the reaction 3A + B + C D + E where the rate law is defined as – = k[A]2[B][C] An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 10–4 M. 74. After 3.00 minutes, [A] = 3.26 10–5 M. The value of k is A) 6.23 10–3 L3/mol3·s B) 3.26 10–5 L3/mol3·s C) 1.15 102 L3/mol3·s D) 1.00 108 L3/mol3·s E) none of these 75. The half-life for this experiment is A) 1.11 102 s B) 87.0 s C) 6.03 10–3 s D) 117 s E) none of these 76. The concentration of C after 10.0 minutes is A) 1.00 M B) 1.10 10–5 M C) 0.330 M D) 0.100 M E) none of these 77. The concentration of A after 10.0 minutes is A) 1.06 10–9 M B) 2.38 10–6 M C) 9.80 10–6 M D) 1.27 10–5 M E) none of these 78. The reaction A B+C is second order in A. When [A]0 = 0.100 M, the reaction is 20.0% complete in 28.7 minutes. Calculate the value of the rate constant (in L/min·mol). A) 8.71 10–2 B) 6.97 10–4 C) 1.96 D) 1.39 E) none of these 79. The reaction A B+C is second order in A. When [A]0 = 0.100 M, the reaction is 20.0% complete in 43.1 minutes. Calculate the half-life for the reaction. A) 1.72 102 min B) 10.8 min C) 2.15 104 min D) 7.66 min E) none of these 80. A first-order reaction is 40.0% complete at the end of 42.6 minutes. What is the value of the rate constant (in min–1)? A) 2.15 10–2 B) 1.20 10–2 C) 83.4 D) 46.5 E) none of these 81. The OH· radical disproportionates according to the elementary chemical reaction This reaction is second order in OH·. The rate constant for the reaction is 2.0 10–12 cm3/molecules at room temperature. If the initial OH· concentration is 1.9 1013 molecules/cm3, what is the first half-life for the reaction? A) 3.5 1011 s B) 3.8 101 s C) 2.6 10–2 s D) 5.3 10–14 s E) 1.3 10–2 s 82. At a particular temperature, N2O5 decomposes according to a first-order rate law with a half-life of 3.0 s. If the initial concentration of N2O5 is 1.0 1016 molecules/cm3, what will be the concentration in molecules/cm3 after 11.9 s? A) 6.4 1014 B) 3.4 101 C) 1.0 1016 D) 1.9 1014 E) 2.3 10–1 83. At a given temperature, a first-order reaction has a rate constant of 2.5 10–3 s–1. The time required for the reaction to be 28% completed is A) 8.5 min B) 0.95 min C) 29 min D) 2.2 min E) 22 min 84. A chemical reaction that is first order in X is observed to have a rate constant of 1.8 10–2s–1. If the initial concentration of X is 1.0 M, what is the concentration of X after 195 s? A) 33 M B) 0.65 M C) 0.22 M D) 0.97 M E) 0.030 M 85. A particular first-order reaction has a rate constant of 0.0485 hr–1. What is the halflife for this reaction? A) 1.00 hr B) 14.3 hr C) 20.6 hr D) 0.0699 hr E) 0.0485 hr 86. The reaction A ? products is first order. If the initial concentration of A is 0.631 M and, after 46.6 seconds have elapsed, the concentration of A has fallen to 0.0307 M, what is the rate constant of the reaction? A) 0.0649 s–1 B) 0.0149 s–1 C) 0.0129 s–1 D) 0.665 s–1 E) 0.0215 s–1 87. The reaction A ? products is second order. If the initial concentration of A is 0.506 M and, after 16.7 seconds have elapsed, the concentration of A has fallen to 0.0528 M, what is the rate constant of the reaction? A) 0.135 M–1 s–1 B) 1.02 M–1 s–1 C) 0.0415 M–1 s–1 D) 0.0271 M–1 s–1 E) 0.0599 M–1 s–1 88. The radioactive nuclide 185Ta undergoes first-order decay with a half-life of 49.4 min. If a quantity of 185Ta is produced, what fraction remains after 75.0 seconds? A) 0.659 B) 0.349 C) 0.0253 D) 0.983 E) 0.0174 89. 63Ni decays by a first-order process via the emission of a beta particle. The 63Ni isotope has a half-life of 100. years. How long will it take for 83% of the nickel to undergo decay? A) 27 years B) 1.2 years C) 110 years D) 12 years E) 260 years Use the following to answer questions 90-91. Two isomers (A and B) of a given compound dimerize as follows: 2A A2 2B B2 Both processes are known to be second order in reactant, and k1 is known to be 0.25 L/mol·s at 25°C, where Rate = – = k1[A]2 In a particular experiment, A and B were placed in separate containers at 25°, where [A]0 = 1.0 10–2 M and [B]0 = 2.5 10–2 M. It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. 90. Calculate the concentration of A2 after 3.0 minutes. A) 2.8 10–22 M B) 6.9 10–3 M C) 3.1 10–3 M D) 1.6 10–3 M E) none of these 91. Calculate the value of k2 where Rate = – = k2[B]2 A) 2.2 L/mol·s B) 0.75 L/mol·s C) 1.9 L/mol·s D) 0.21 L/mol·s E) none of these 92. Two isomers (A and B) of a given compound dimerize as follows: 2A A2 2B B2 Both processes are known to be second order in reactant, and k1 is known to be 0.29 L/mol·s at 25°C, where: In a particular experiment, A and B were placed in separate containers at 25oC, where [A]0 = 1.0 10–2 M and [B]0 = 2.5 10–2 M. It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. Calculate the half-life for the reaction involving A. A) 3.4 102 s B) 1.0 102 s C) 1.8 102 s D) 2.9 103 s E) none of these 93. The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics. Assuming the form of the rate law is: where k = 3.4 10–5 s–1 at 25°C, what is the initial rate of reaction at 25°C where [N2O5]0 = 6.4 10–2 M? A) 3.4 10–5 mol/L·s B) 2.2 10-6 mol/L·s C) 5.3 10–4 mol/L·s D) 6.4 10–2 mol/L·s E) none of these 94. The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics. Assuming the form of the rate law is: where k = 3.4 10–5 s–1 at 25°C, what is the half-life for the reaction described? A) 2.9 104 s B) 2.0 104 s C) 8.7 108 s D) 4.9 10–5 s E) none of these 95. Consider a reaction of the type aA Products in which the rate law is found to be rate = k[A]3 (yes, a termolecular reaction is improbable but possible). If the first half-life of the reaction is found to be 40 seconds, what is the time for the second half-life? A) 10 seconds B) 20 seconds C) 80 seconds D) 160 seconds E) 320 seconds 96. The reaction 2NO2 2NO + O2 obeys the rate law: at 500. K. If the initial concentration of NO2 is 1.00 M, how long will it take for the [NO2] to decrease to 14.2% of its initial value? A) 61.3 s B) 139 s C) 432 s D) 1.40 10–2 s E) cannot be determined from this data 97. If the reaction 2HI H2 + I2 is second order, which of the following will yield a linear plot? A) log [HI] vs time B) 1/[HI] vs time C) [HI] vs time D) ln [HI] vs time E) None of these. 98. The reaction 3NO N2O + NO2 is found to obey the rate law, Rate = k[NO]2. If the first half-life of the reaction is found to be 2.0 s, what is the length of the fourth half-life? A) 2.0 s B) 4.0 s C) 8.0 s D) 12.0 s E) 16.0 s 99. In 6 M HCl, the complex ion Ru(NH3)63+ decomposes to a variety of products. The reaction is first order in Ru(NH3)63+ and has a half-life of 14 hours at 25°C. Under these conditions, how long will it take for the [Ru(NH3)63+] to decrease to 13.4% of its initial value? A) 2.9 hours B) 9.7 hours C) 1.9 hours D) 14 hours E) 41 hours 100. The elementary chemical reaction O + ClO Cl + O2 is made pseudo-first order in oxygen atoms by using a large excess of ClO radicals. The rate constant for the reaction is 2.6 cm3/molecules. If the initial concentration of ClO is 1.0 1011 molecules/cm3, how long will it take for the oxygen atoms to decrease to 10.% of their initial concentration? A) 1.1 s B) 0.041 s C) 0.27 s D) 0.89 s E) 2.7 s 101. Determine the molecularity of the following elementary reaction: O3 O2 + O. A) unimolecular B) bimolecular C) termolecular D) quadmolecular E) molecularity cannot be determined 102. The decomposition of ozone may occur through the two-step mechanism shown: step 1 O3 Æ O2 + O step 2 O3 + O Æ 2O2 The oxygen atom is considered to be a(n) A) reactant B) product C) catalyst D) reaction intermediate E) activated complex 103. The rate law for a reaction is found to be Rate = k[A]2[B]. Which of the following mechanisms gives this rate law? I. A+B E (fast) E + B Æ C + D (slow) II. A + B E (fast) E + A Æ C + D (slow) III. A + A Æ E (slow). E + B Æ C + D (fast) A) I B) II C) III D) two of these E) none of these 104. The experimental rate law for the decomposition of nitrous oxide (N2O) to N2 and O2 is Rate = k[N2O]2. Two mechanisms are proposed: I. N2O Æ N2 + O N2O + O Æ N2 + O2 II. 2N2O N4O2 N4O2 Æ 2N2 + O2 Which of the following could be a correct mechanism? A) Mechanism I, with the first step as the rate-determining step. B) Mechanism I, with the second step as the rate-determining step as long as the first step is a fast equilibrium step. C) Mechanism II, with the second step as the rate-determining step if the first step is a fast equilibrium step. D) None of the choices (A-C) could be correct. E) At least two of the above choices (A-C) could be correct. 105. Consider the reaction 2O3(g) 3O2(g). The following mechanism is proposed: O3 O2 + O O3 + O 2O2 If we assume the second step of the mechanism is the rate determining step and the first step is a fast equilibrium step, which of the following rate laws is predicted by this mechanism? A) rate = k[O3] B) rate = k[O3]2[O2] C) rate = k[O3]2[O2]–1 D) rate = k[O3]2 E) none of these 106. Of what use is it to find a rate law for a reaction? A) We can use the rate law to directly determine coefficients in the balanced equation. B) From the rate law we can evaluate potential reaction mechanisms. C) The rate law gives us a good indication of the thermodynamic stability of the products. D) The rate law can lead us to determine the equilibrium constant for the reaction. E) None of these. Use the following to answer questions 107-110. The following questions refer to the reaction 2A2 + B2 2C. The following mechanism has been proposed: step 1 (very slow) A2 + B2 R + C step 2 (slow) A2 + R C 107. What is the molecularity of step 2? A) unimolecular B) bimolecular C) termolecular D) quadmolecular E) molecularity cannot be determined 108. Which step is rate determining? A) both steps B) step 1 C) step 2 D) a step that is intermediate to step 1 and step 2 E) none of these 109. According to collision theory, the activated complex that forms in step 1 could have which of the following structures? (The dotted lines represent partial bonds.) A) B) C) D) E) 110. According to the proposed mechanism, what should the overall rate law be? A) rate = k[A2]2 B) rate = k[A2] C) rate = k[A2][B2] D) rate = k[A2][R] E) rate = k[R]2 Use the following to answer questions 111-113. Under certain conditions the reaction H2O2 + 3I– + 2H+ I3– + 2H2O occurs by the following series of steps: k1 Step 1. H2O2+ H+ H3O2+ k–1 Step 2. Step 3. Step 4. Step 5. H3O2+ + I– H2O + HOI HOI + I– OH– + I2 OH– + H+ H2O I2 + I– I3– (slow, rate constant k2) (fast, rate constant k3) (fast, rate constant k4) (fast, rate constant k5) 111. Which of the steps would be called the rate-determining step? A) 1 B) 2 C) 3 D) 4 E) 5 112. The rate constant k for the reaction would be given by A) k = k2 B) k = k2k3 C) k = k2K D) k = k5 E) k = Kk2k3k4k5 113. The rate law for the reaction would be: A) [I3]/t = k[H2O2] B) [I3]/t = k[H2O2][H+][I–] C) [I3]/t = k[H2O2][H+] D) [I3]/t = k[H2O2][I–] E) [I3]/t = k[H2O2][H+]2[I–]–3 114. The reaction: 2A + B Æ C has the following proposed mechanism: Step 1: A + B D (fast equilibrium) Step 2: D + B Æ E Step 3: E + A Æ C + B If step 2 is the rate-determining step, then the rate of formation of C should equal: A) k[A] B) k[A]2[B] C) k[A]2[B]2 D) k[A][B] E) k[A][B]2 115. The reaction 2NO + O2 2NO2 obeys the rate law – = kobsd[NO]2[O2]. Which of the following mechanisms is consistent with the experimental rate law? A) NO + NO Æ N2O2 (slow) N2O2 + O2 Æ 2NO2 (fast) B) NO + O2 NO3 NO3 + NO Æ 2NO2 C) 2NO N2O2 N2O2 Æ NO2 + O NO + O Æ NO2 (fast equilibrium) (slow) (fast equilibrium) (slow) (fast) D) O2 + O2 O2 + O2 O2 + NO NO2 + O O + NO NO2 (slow) (fast) (fast) E) none of these 116. The rate constant k is dependent on I. II. III. IV. the order of the reaction A) none of these B) one of these C) two of these D) three of these E) all of these the concentration of the reactant the nature of the reactants the temperature Use the following to answer questions 117-119. The questions below refer to the following diagram: 117. Why is this reaction considered to be exothermic? A) Because energy difference B is greater than energy difference C. B) Because energy difference B is greater than energy difference A. C) Because energy difference A is greater than energy difference C. D) Because energy difference B is greater than energy difference C plus energy difference A. E) Because energy difference A and energy difference C are about equal. 118. At what point on the graph is the activated complex present? A) point W B) point X C) point Y D) point Z E) none of these 119. If the reaction were reversible, would the forward or the reverse reaction have a higher activation energy? A) The diagram shows no indication of any activation energy. B) The forward and reverse activation energies are equal. C) The forward activation energy would be greater. D) The reverse activation energy would be greater. E) None of these. 120. What would happen if the kinetic energy of the reactants was not enough to provide the needed activation energy? A) The products would be produced at a lower energy state. B) The rate of the reaction would tend to increase. C) The activated complex would convert into products. D) The reactants would continue to exist in their present form. E) The products would form at an unstable energy state. 121. The rate constant for a reaction at 40.0°C is exactly 5 times that at 20.0°C. Calculate the Arrhenius energy of activation for the reaction. A) 13.4 kJ/mol B) 7.38 kJ/mol C) 61.4 kJ/mol D) 5.00 kJ/mol E) none of these Use the following to answer questions 122-124. Use the potential energy diagram shown to answer the following: 122. Which letter shows the activation energy (without use of a catalyst)? A) a B) b C) c D) d E) e 123. Which letter shows the change in energy for the overall reaction? A) a B) b C) c D) d E) e 124. Which letter shows the activation energy using a catalyst? A) a B) b C) c D) d E) e Use the following to answer questions 125-126. The questions below refer to the following information: The rate constant k for the reaction shown below is 2.6 10–8 L/mol s when the reaction proceeds at 300.0 K. The activation energy is 98000 J/mol. (The universal gas constant, R, is 8.314 J/mol·K) 2NOCl Æ 2NO + Cl2 125. Determine the magnitude of the frequency factor for the reaction. A) 1.2 108 B) 4.6 109 C) 3.0 109 D) 2.7 108 E) 9.1 109 126. If the temperature changed to 310 K, the rate constant k would change. The ratio of k at 310 K to k at 300.0 K is closest to what whole number? A) 1 B) 2 C) 3 D) 4 E) 5 127. Use the following information to determine the activation energy for the reaction shown here: 2NO N2 + O2 Temperature (K) Rate Constant (L/mol·s) 1400 0.143 1500 0.703 A) 6.2 104 J/mol B) 1.3 101 J/mol C) 9.6 103 J/mol D) 3.3 104 J/mol E) 2.8 105 J/mol 128. When ethyl chloride, CH3CH2Cl, is dissolved in 1.0 M NaOH, it is converted into ethanol, CH3CH2OH, by the reaction: CH3CH2Cl + OH– CH3CH2OH + Cl– At 25°C the reaction is first order in CH3CH2Cl, and the rate constant is 4.7 10– 3 s–1. If the activation parameters are A = 3.4 1014 s–1 and Ea = 100.0 kJ/mol, what will the rate constant be at 40.°C? A) 3.3 10–2 s–1 B) 6.8 10–4 s–1 C) 1.5 103 s–1 D) 9.1 10–3 s–1 E) 2.4 10–3 s–1 129. Which of the following statements best describes the condition(s) needed for a successful formation of a product according to the collision model? A) The collision must involve a sufficient amount of energy, provided from the motion of the particles, to overcome the activation energy. B) The relative orientation of the particles has little or no effect on the formation of the product. C) The relative orientation of the particles has an effect only if the kinetic energy of the particles is below some minimum value. D) The relative orientation of the particles must allow for formation of the new bonds in the product. E) The energy of the incoming particles must be above a certain minimum value, and the relative orientation of the particles must allow for formation of new bonds in the product. 130. The rate constant for a reaction is 1.7 10–2 s–1 at 662 K and 5.0 10–2 s–1 at 848 K. What is the activation energy? A) 12 kJ/mol B) 27 kJ/mol C) 640 kJ/mol D) 3300 kJ/mol E) This can't be solved without knowing the frequency factor. 131. For the second-order reaction NO(g) + O3(g) ? NO2(g) + O2(g), the rate constant has been measured to be 1.08 * 107 M–1 s–1 at 298 K and the activation energy has been measured to be 11.4 kJ/mol over the temperature range 195 K to 304 K. What is the rate constant at 281 K? (R = 8.3145 J K–1 mol–1) A) 1.08 * 107 M–1 s–1 B) 1.08 * 109 M–1 s–1 C) 8.18 * 106 M–1 s–1 D) 1.43 * 107 M–1 s–1 E) 9.51 * 105 M–1 s–1 132. The reaction 2H2O2 2H2O + O2 has the following mechanism: H2O2 + I– Æ H2O + IO– H2O2 + IO– Æ H2O + O2 + I– The catalyst in the reaction is: A) H2O B) I– C) H2O2 D) IO– E) There is no catalyst in this reaction. 133. Which of the following statements is typically true for a catalyst? I. The concentration of the catalyst will go down as a reaction proceeds. II. The catalyst provides a new pathway in the reaction mechanism. III. The catalyst speeds up the reaction. A) I only B) II only C) III only D) I and III E) II and III 134. The catalyzed pathway in a reaction mechanism has a __________ activation energy and thus causes a __________ reaction rate. A) higher, lower B) higher, higher C) lower, higher D) lower, steady E) higher, steady 135. Which of the following statements about enzymes is incorrect? A) They are proteins that catalyze specific biologic reactions. B) Several hundred are now known. C) The molecules they react with are called substrates. D) They are equal to inorganic catalysts in efficiency. E) All of these are correct. 136. Determine (a) the rate equation and (b) the rate constant for the hypothetical reaction A + B C given the following initial concentrations and initial rate data. [A]0 [B]0 Initial Rate Run # (mol/L) (mol/L) (mol/L·s) (1) 0.100 0.100 0.18 (2) 0.100 0.200 0.36 (3) 0.200 0.200 1.44 Use the following to answer questions 137-139. A reaction represented by the equation was studied at a specific temperature and the following data were collected: 3O2 (g) Æ 2O3 (g) Time (seconds) 0 46.89 98.82 137.9 200.0 286.9 337.9 511.3 Total pressure (atm) 1.000 0.9500 0.9033 0.8733 0.8333 0.7900 0.7700 0.7233 137. What is the rate law for this reaction? 138. What is the value of the rate constant? 139. How many seconds would it take for the total pressure to be 0.7133 atm? Use the following to answer questions 140-143. For the reaction aA Products, use the following choices a) zero order in A b) first order in A c) second order in A d) a, b, c e) none of the above 140. The half-life is constant. 141. A plot of [A] vs. t is a straight line. 142. [A] is constant. 143. The rate is constant over time. 144. Which of the following is true about a system at equilibrium? A) The concentration(s) of the reactant(s) is equal to the concentration(s) of the product(s). B) No new product molecules are formed. C) The concentration(s) of reactant(s) is constant over time. D) The rate of the reverse reaction is equal to the rate of the forward reaction and both rates are equal to zero. E) None of the above (A-D) is true. 145. Which of the following is true about chemical equilibrium? A) It is microscopically and macroscopically static. B) It is microscopically and macroscopically dynamic. C) It is microscopically static and macroscopically dynamic. D) It is microscopically dynamic and macroscopically static. E) None of these are true about chemical equilibrium. 146. Equilibrium is reached in chemical reactions when: A) The rates of the forward and reverse reactions become equal. B) The concentrations of reactants and products become equal. C) The temperature shows a sharp rise. D) All chemical reactions stop. E) The forward reaction stops. 147. For a particular system at a particular temperature there ______ equilibrium constant(s) and there _______ equilibrium position(s). A) are infinite; is one B) is one; are infinite C) is one; is one D) are infinite; are infinite E) none of these 148. For the reaction given below, 2.00 moles of A and 3.00 moles of B are placed in a 6.00-L container. At equilibrium, the concentration of A is 0.231 mol/L. What is the concentration of B at equilibrium? A) 0.231 mol/L B) 0.295 mol/L C) 0.500 mol/L D) 0.462 mol/L E) none of these 149. The value of the equilibrium constant, K, is dependent on: I. the temperature of the system II. III. IV. the nature of the reactants and products the concentration of the reactants the concentration of the products A) I, II B) II, III C) III, IV D) It is dependent on three of the above choices. E) It is not dependent on any of the above choices. 150. If the equilibrium constant for A + B C is 0.213, then the equilibrium constant for 2C 2A + 2B is A) 0.574 B) 4.69 C) 0.426 D) 22.0 E) 0.213 151. Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g) A) [X]2[Y][W]3[V] B) C) D) E) none of these 152. If, at a given temperature, the equilibrium constant for the reaction H2(g) + Cl2(g) 2HCl(g) is Kp, then the equilibrium constant for the reaction HCl(g) H2(g) + Cl2 (g) can be represented as: A) B) Kp2 C) D) E) none of these 153. Apply the law of mass action to determine the equilibrium expression for 2NO2Cl(aq) 2NO2(aq) + Cl2(aq). A) K = 2[NO2][Cl2]/2[NO2Cl] B) K = 2[NO2Cl]/2[NO2][Cl2] C) K = [NO2Cl]2/[NO2]2[Cl2] D) K = [NO2]2[Cl2]/[NO2Cl]2 E) K = [NO2Cl]2[NO2]2[Cl2] 154. At a given temperature, K = 0.030 for the equilibrium: PCl5(g) PCl3(g) + Cl2(g) What is K for: Cl2(g) + PCl3(g) PCl5(g)? A) 0.030 B) 33 C) 0.00090 D) 30. E) 1100 155. Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g) 2Cu2O(s), K1 2CuO(s) Cu2O(s) + ?O2(g), K2 what is K for the system 2Cu(s) + O2(g) 2CuO(s) equivalent to? A) (K1)(K2) B) (K2)?/(K1) C) (K1)?/(K2) D) (K2)?/(K1) E) (K1)(K2)? 156. Which expression correctly describes the equilibrium constant for the following reaction? 2C2H2(g) + 5O2(g) 4CO2(g) + 2H2O(g) A) K = ( 2[C2H2] + 5[O2] ) / ( 4[CO2] + 2[H2O] ) B) K = ( 4[CO2] + 2[H2O] ) / (2[C2H2] + 5[O2] ) C) K = ( [CO2][H2O] ) / ( [C2H2][O2] ) D) K = ( [CO2]4[H2O]2 ) / ( [C2H2]2[O2]5 ) E) K = ( [C2H2]2[O2]5 ) / ( [CO2]4[H2O]2 ) Use the following to answer questions 157-158. Consider the chemical system CO + Cl2 COCl2; K = 4.6 109 L/mol. 157. How do the equilibrium concentrations of the reactants compare to the equilibrium concentration of the product? A) They are much smaller. B) They are much bigger. C) They are about the same. D) They have to be exactly equal. E) You can't tell from the information given. 158. If the concentration of the product were to double, what would happen to the equilibrium constant? A) It would double its value. B) It would become half its current value. C) It would quadruple its value. D) It would not change its value. E) It would depend on the initial conditions of the product. 159. Determine the equilibrium constant for the system N2O4 2NO2 at 25°C. The concentrations are shown here: [N2O4] = 2.93 10–2 M, [NO2] = 1.41 10–2 M. A) 0.481 B) 2.08 C) 1.47 102 D) 0.232 E) 6.79 10–3 160. If K = 0.138 for A2 + 2B 2AB, then for 4AB 2A2 + 4B, K would equal: A) 0.276 B) 0.138 C) –0.138 D) 3.62 E) 52.5 161. Consider the gaseous reaction CO(g) + Cl2(g) COCl2(g). What is the expression for Kp in terms of K? A) K(RT) B) K/(RT) C) K(RT)2 D) K/(RT)2 E) 1/K(RT) 162. For the reaction N2O4(g) 2NO2(g), Kp = 0.148 at a temperature of 298 K. What is Kp for the following reaction? 4NO2(g) 2N2O4(g) A) 6.76 B) 0.296 C) 3.38 D) 4.57 * 101 E) 2.19 * 10–2 163. For the reaction H2(g) + Cl2(g) 2HCl(g), Kc = 3.36 * 1023 at a temperature of 429 K. What is Kp at this temperature? A) 3.36 * 1023 B) 1.18 * 1025 C) 9.55 * 1021 D) 4.17 * 1026 E) 2.71 * 1020 164. For the reaction NO(g) + ?O2(g) NO2(g) at 750°C, the equilibrium constant Kc equals: A) 1.0 B) Kp C) Kp(RT)–? D) Kp(RT)? E) Kp(RT)? 165. An equilibrium reaction, A2(g) + 3B2(g) 2C(g), has a Kp at 225ºC of 1.5 10–3 /atm2. What is K for this reaction at that temperature? A) 9.0 10–7 B) 4.4 10–6 C) 2.5 D) 3.7 10–5 E) 0.51 166. Find the value of the equilibrium constant (K) (at 500 K) for N2(g) + 3H2(g) 2NH3(g). The value for Kp at 500 K is 1.5 10–5/atm2. A) 7.5 10–2 B) 1.3 10–2 C) 9.6 10–2 D) 2.5 10–2 E) 6.0 10–2 167. Consider the following reaction: CS2(g) + 4H2(g) CH4(g) + 2H2S(g). The equilibrium constant K is about 0.26 at 900.°C. What is Kp at this temperature? A) 2.4 103 B) 2.8 10–5 C) 2.8 10–5 D) 2.5 101 E) 1.1 10–3 168. Given the equation 2NOCl2(g) 2NO(g) + Cl2(g), the equilibrium constant is about 0.0290 at 115°C. Calculate Kp. A) 0.0290 B) 0.923 C) 0.274 D) 29.4 E) none of these 169. Calculate Kp for using the following data: Kp = 2.4 106 Kp = 1.8 1037 A) 4.3 1043 B) 2.2 1043 C) 3.2 10–25 D) 5.7 10–13 E) 1.0 10–12 170. Consider the reaction: CaCl2(s) + 2H2O(g) CaCl2·2H2O(s) The equilibrium constant for the reaction as written is: A) K = B) C) D) K = [H2O]2 E) K = 171. Consider the reaction At 1273 K, the Kp value is 167.5. What is the at equilibrium if the is 0.25 atm at this temperature? A) 3.2 atm B) 0.13 atm C) 13 atm D) 6.5 atm E) 9.2 atm Use the following to answer questions 172-174. Consider the following equilibrium: H2(g) + I2(s) 2HI(g) 172. The proper Keq expression is: A) B) C) D) E) 173. Which of the following statements about the equilibrium is false? A) If the system is heated, the right side is favored. B) This is a heterogeneous equilibrium. C) If the pressure on the system is increased by changing the volume, the left side is favored. D) Adding more H2(g) increases the equilibrium constant. E) Removing HI as it forms forces the equilibrium to the right. 174. Consider the reaction: 2SO2(g) + O2(g) 2SO3(g) at constant temperature. Initially a container is filled with pure SO3(g) at a pressure of 2 atm, after which equilibrium is reached. If y is the partial pressure of O2 at equilibrium, the value of Kp is: A) B) C) D) E) none of these 175. Which of the following is true for a system whose equilibrium constant is relatively small? A) It will take a short time to reach equilibrium. B) It will take a long time to reach equilibrium. C) The equilibrium lies to the left. D) The equilibrium lies to the right. E) Two of these. 176. The reaction quotient for a system is 7.2 102. If the equilibrium constant for the system is 36, what will happen as equilibrium is approached? A) There will be a net gain in product. B) There will be a net gain in reactant. C) There will be a net gain in both product and reactant. D) There will be no net gain in either product or reactant. E) The equilibrium constant will decrease until it equals the reaction quotient. 177. Consider the following reaction: 2HF(g) H2(g) + F2(g) (K = 1.00 10–2) Given 1.00 mole of HF(g), 0.316 mole of H2(g), and 0.750 mole of F2(g) are mixed in a 5.00-L flask, determine the reaction quotient, Q. A) Q = 0.0474 B) Q = 0.237 C) Q = 0.0593 D) Q = 2.07 E) none of these Use the following to answer questions 178-179. Nitric oxide, an important pollutant in air, is formed from the elements nitrogen and oxygen at high temperatures, such as those obtained when gasoline burns in an automobile engine. At 2000°C, K for the reaction N2(g) + O2(g) 2NO(g) is 0.01. 178. Predict the direction in which the system will move to reach equilibrium at 2000°C if 0.4 moles of N2, 0.1 moles of O2, and 0.08 moles of NO are placed in a 1.0-liter container. A) The system remains unchanged. B) The concentration of NO will decrease; the concentrations of N2 and O2 will increase. C) The concentration of NO will increase; the concentrations of N2 and O2 will decrease. D) The concentration of NO will decrease; the concentrations of N2 and O2 will remain unchanged. E) More information is necessary. 179. A 1-L container originally holds 0.4 mol of N2, 0.1 mol of O2, and 0.08 mole of NO. If the volume of the container holding the equilibrium mixture of N2, O2, and NO is decreased to 0.5 L without changing the quantities of the gases present, how will their concentrations change? A) The concentration of NO will increase; the concentrations of N2 and O2 will decrease. B) The concentrations of N2 and O2 will increase; and the concentration of NO will decrease. C) The concentrations of N2, O2, and NO will increase. D) The concentrations of N2, O2, and NO will decrease. E) There will be no change in the concentrations of N2, O2, and NO. 180. Consider the following equilibrated system: 2NO2(g) 2NO(g) + O2(g). If the Kp value is 0.884, find the equilibrium pressure of the O2 gas if the NO2 gas pressure is 0.520 atm and the PNO is 0.300 atm at equilibrium. A) 1.53 atm B) 36.3 atm C) 0.510 atm D) 0.294 atm E) 2.66 atm 181. For the reaction given below, 2.00 moles of A and 3.00 moles of B are placed in a 6.00-L container. A(g) + 2B(g) C(g) At equilibrium, the concentration of A is 0.235 mol/L. What is the value of K? A) 1.38 B) 1.07 C) 0.235 D) 4.55 E) 0.418 182. A 10.0-g sample of solid NH4Cl is heated in a 5.00-L container to 900.°C. At equilibrium the pressure of NH3(g) is 1.14 atm. NH4Cl(s) NH3(g) + HCl(g) The equilibrium constant, Kp, for the reaction is: A) 1.14 B) 1.30 C) 2.28 D) 4.68 E) none of these 183. Consider the reaction H2 + I2 2HI for which K = 42.1 at a high temperature. If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium, determine the equilibrium concentration of the hydrogen. A) 1.1 10–1 M B) 7.7 10–2 M C) 3.9 10–2 M D) 1.3 101 M E) 5.9 10–3 M 184. Consider the equation A(aq) + 2B(aq) 3C(aq) + 2D(aq). In one experiment, 45.0 mL of 0.050 M A is mixed with 25.0 mL 0.100 M B. At equilibrium the concentration of C is 0.0410 M. Calculate K. A) 7.3 B) 0.34 C) 0.040 D) 0.14 E) none of these 185. The reaction: H2(g) + I2(g) 2HI(g) has Kp = 45.9 at 763 K. A particular equilibrium mixture at that temperature contains gaseous HI at a partial pressure of 4.00 atm and hydrogen gas at a partial pressure of 0.215 atm. What is the partial pressure of I2? A) 0.215 atm B) 0.405 atm C) 1.62 atm D) 10.9 atm E) 74.4 atm 186. For the equilibrium system: CO2(g) + H2(g) CO(g) + H2O(g) H = +42 kJ/mol K equals 1.6 at 1260 K. If 0.15 mol each of CO2, H2, CO, and H2O (all at 1260 K) were placed in a 1.0-L thermally insulated vessel that was also at 1260 K, then as the system came to equilibrium: A) The temperature would decrease and the mass of CO2 would increase. B) The temperature would decrease and the mass of CO2 would decrease. C) The temperature would remain constant and the mass of CO2 would increase. D) The temperature would increase and the mass of CO2 would increase. E) The temperature would increase and the mass of CO2 would decrease. 187. CS2(g) + 3Cl2(g) CCl4(g) + S2Cl2(g) At a given temperature, the reaction above is at equilibrium when [CS2] = 0.050 M, [Cl2] = 0.25 M, [CCl4] = 0.15 M, and [S2Cl2] = 0.35 M. What would be the direction of the reaction when the reactants and products have the following concentrations: CS2 = 0.14 M, Cl2 = 0.22 M, CCl4 = 0.28 M, and S2Cl2 = 0.37 M? A) to the right B) to the left C) no change D) cannot predict unless we know the temperature E) cannot predict unless we know whether the reaction is endothermic or exothermic 188. A mixture of nitrogen and hydrogen was allowed to come to equilibrium at a given temperature. 3H2 + N2 2NH3 An analysis of the mixture at equilibrium revealed 1.8 mol N2, 3.0 mol H2, and 1.8 mol NH3. How many moles of H2 were present at the beginning of the reaction? A) 3.0 B) 4.5 C) 4.8 D) 5.7 E) 4.2 189. Carbon disulfide and chlorine react according to the following equation: CS2(g) + 3Cl2(g) S2Cl2(g) + CCl4(g) When 2.60 mol of CS2 and 4.25 mol of Cl2 are placed in a 2.00-L container and allowed to come to equilibrium, the mixture is found to contain 0.400 mol of CCl4. How many moles of Cl2 are present at equilibrium? A) 2.200 mol B) 0.400 mol C) 3.05 mol D) 3.45 mol E) 1.52 mol 190. Initially 2.0 moles of N2(g) and 4.0 moles of H2(g) were added to a 1.0-liter container and the following reaction then occurred: 3H2(g) + N2(g) 2NH3(g) The equilibrium concentration of NH3(g) = 0.62 moles/liter at 700.°C. The value for K at 700.°C for the formation of ammonia is: A) 1.2 10–1 B) 7.4 10–2 C) 7.9 10–3 D) 3.8 10–1 E) none of these Use the following to answer questions 191-192. Consider the following reaction (assume an ideal gas mixture): 2NOBr(g) 2NO(g) + Br2(g) A 1.0-liter vessel was initially filled with pure NOBr, at a pressure of 4.0 atm, at 300 K. 191. After equilibrium was established, the partial pressure of NOBr was 3.1 atm. What is Kp for the reaction? A) 0.26 B) 0.038 C) 0.13 D) 0.45 E) none of these 192. After equilibrium was reached, the volume was increased to 2.0 liters, while the temperature was kept at 300 K. The result of this change was A) an increase in Kp B) a decrease in Kp C) a shift in the equilibrium position to the right D) a shift in the equilibrium position to the left E) none of these 193. Nitrogen gas (N2) reacts with hydrogen gas (H2) to form ammonia (NH3). At 200°C in a closed container, 1.0 atm of nitrogen gas is mixed with 2.0 atm of hydrogen gas. At equilibrium, the total pressure is 2.1 atm. Calculate the partial pressure of hydrogen gas at equilibrium. A) 2.1 atm B) 0.65 atm C) 1.5 atm D) 0.0 atm E) none of these 194. The following reaction is investigated (assume an ideal gas mixture): 2N2O(g) + N2H4(g) 3N2(g) + 2H2O(g) Initially there are 0.10 moles of N2O and 0.25 moles of N2H4, in a 10.0-L container. If there are 0.064 moles of N2O at equilibrium, how many moles of N2 are present at equilibrium? A) 1.8 10–2 B) 3.6 10–2 C) 5.4 10–2 D) 1.1 10–1 E) none of these 195. A 3.00-liter flask initially contains 3.00 mol of gas A and 1.50 mol of gas B. Gas A decomposes according to the following reaction: 3A 2B + C The equilibrium concentration of gas C is 0.116 mol/L. Determine the equilibrium concentration of gas A. A) 0.116 M B) 0.652 M C) 0.732 M D) 0.884 M E) 0.348 M 196. A 3.00-liter flask initially contains 3.00 mol of gas A and 1.50 mol of gas B. Gas A decomposes according to the following reaction: 3A 2B + C The equilibrium concentration of gas C is 0.120 mol/L. Determine the equilibrium concentration of gas B. A) 0.120 M B) 0.620 M C) 0.740 M D) 0.260 M E) 0.240 M 197. A 3.00-liter flask initially contains 3.00 mol of gas A and 1.50 mol of gas B. Gas A decomposes according to the following reaction: 3A 2B + C The equilibrium concentration of gas C is 0.147 mol/L. Determine the value of the equilibrium constant, K. A) 0.209 B) 0.166 C) 3.89 10–3 D) 0.531 E) none of these 198. A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed explosively according to the following equation: NH4NO3(s) N2O(g) + 2H2O(g) At equilibrium the total pressure in the container was found to be 2.72 atm at a temperature of 500.°C. Calculate Kp. A) 0.822 B) 1.64 C) 0.745 D) 2.98 E) 80.5 Use the following to answer questions 199-202. Given the equation 2A(g) 2B(g) + C(g). At a particular temperature, K = 1.6 104. 199. If you mixed 5.0 mol B, 0.10 mol C, and 0.0010 mol A in a one-liter container, which direction would the reaction initially proceed? A) To the left. B) To the right. C) The above mixture is the equilibrium mixture. D) Cannot tell from the information given. E) None of these (A-D). 200. Addition of chemical B to an equilibrium mixture of the above will A) cause [A] to increase B) cause [C] to increase C) have no effect D) cannot be determined E) none of the above 201. At a higher temperature, K = 1.8 10–5. Placing the equilibrium mixture in an ice bath (thus lowering the temperature) will A) cause [A] to increase B) cause [B] to increase C) have no effect D) cannot be determined E) none of the above 202. Raising the pressure by lowering the volume of the container will A) cause [A] to increase B) cause [B] to increase C) have no effect D) cannot be determined E) none of the above Use the following to answer questions 203-204. Consider the following equilibrium: 2NOCl(g) 2NO(g) + Cl2(g) with K = 1.6 10–5. In an experiment, 1.00 mole of pure NOCl and 1.00 mole of pure Cl2 are placed in a 1.00-L container. 203. If x moles of NOCl react, what is the equilibrium concentration of NO? A) x B) 2x C) –x D) –2x E) x2 204. If x moles of NOCl react, what is the equilibrium concentration of Cl2? A) x B) ? x C) 1 + x D) 1 + ? x E) 1 + 2x 205. At a certain temperature K for the reaction 2NO2 N2O4 is 7.5 liters/mole. If 2.0 moles of NO2 are placed in a 2.0-liter container and permitted to react at this temperature, calculate the concentration of N2O4 at equilibrium. A) 0.39 moles/liter B) 0.65 moles/liter C) 0.82 moles/liter D) 7.5 moles/liter E) none of these 206. Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and is allowed to reach equilibrium described by the equation N2O4(g) 2NO2(g) If at equilibrium the N2O4 is 29% dissociated, what is the value of the equilibrium constant, Kc, for the reaction under these conditions? A) 0.82 B) 0.47 C) 2.1 D) 0.34 E) 0.12 207. At 500.0 K, one mole of gaseous ONCl is placed in a one-liter container. At equilibrium it is 5.0% dissociated according to the equation shown here: 2ONCl 2NO + Cl2. Determine the equilibrium constant. A) 6.9 10–5 B) 1.4 10–3 C) 5.3 10–2 D) 9.5 10–1 E) 1.4 104 208. Consider the following equilibrium: 2NOCl(g) 2NO(g) + Cl2(g) with K = 1.6 10–5. 1.00 mole of pure NOCl and 0.920 mole of pure Cl2 are placed in a 1.00-L container. Calculate the equilibrium concentration of NO(g). A) 2.09 10–3 M B) 9.20 10–1 M C) 1.09 M D) 5.90 10–3 M E) 4.17 10–3 M 209. Consider the following equilibrium: 2NOCl(g) 2NO(g) + Cl2(g) with K = 1.6 10–5. 1.00 mole of pure NOCl and 0.985 mole of pure Cl2 are placed in a 1.00-L container. Calculate the equilibrium concentration of Cl2(g). A) 1.6 10–5 M B) 0.987 M C) 0.494 M D) 2.02 10–3 M E) 4.03 10–3 M 210. For the reaction below, Kp = 1.16 at 800.°C. CaCO3(s) CaO(s) + CO2(g) If a 38.9-gram sample of CaCO3 is put into a 10.0-L container and heated to 800.°C, what percent of the CaCO3 will react to reach equilibrium? A) 17.5% B) 33.9% C) 45.5% D) 100.0% E) none of these 211. At –80°C, K for the reaction N2O4(g) 2NO2(g) is 4.66 10–8. We introduce 0.042 mole of N2O4 into a 1.0-L vessel at –80°C and let equilibrium be established. The total pressure in the system at equilibrium will be: A) 0.28 atm B) 0.67 atm C) 1.2 atm D) 0.042 atm E) none of these 212. The equilibrium system 2A 2B + C has a very small equilibrium constant: K = 2.6 10–6. Initially 3.0 moles of A are placed in a 1.5-L flask. Determine the concentration of C at equilibrium. A) 0.011 M B) 0.024 M C) 0.032 M D) 0.048 M E) 2.0 M 213. Which of the following statements concerning equilibrium is not true? A) A system that is disturbed from an equilibrium condition responds in a manner to restore equilibrium. B) Equilibrium in molecular systems is dynamic, with two opposing processes balancing one another. C) The value of the equilibrium constant for a given reaction mixture is the same regardless of the direction from which equilibrium is attained. D) A system moves spontaneously toward a state of equilibrium. E) The equilibrium constant is independent of temperature. Use the following to answer questions 214-216. The questions below refer to the following system: Co(H2O)62+ + 4 Cl– CoCl42– + 6H2O (pink) (blue) When cobalt(II) chloride is added to pure water, the Co2+ ions hydrate. The hydrated form then reacts with the Cl– ions to set up the equilibrium shown here. 214. Which statement below describes the change that the system will undergo if hydrochloric acid is added? A) It should become more blue. B) It should become more pink. C) The equilibrium will shift to the right. D) The equilibrium will shift to the left. E) Two of these. 215. Which statement below describes the change that the system will undergo if water is added? A) More chloride ions will be produced. B) More water will be produced. C) The equilibrium will shift to the right. D) The color will become more blue. E) There will be less of the hydrated cobalt ion at the new equilibrium position. 216. Which statement below describes the change that the system will undergo if silver nitrate is added? A) It should become more blue. B) It should become more pink. C) Water will be produced. D) The silver ion will react with the CoCl42–. E) Nothing will change. Use the following to answer questions 217-219. The following questions refer to the equilibrium shown here: 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g) 217. What would happen to the system if oxygen were added? A) More ammonia would be produced. B) More oxygen would be produced. C) The equilibrium would shift to the right. D) The equilibrium would shift to the left. E) Nothing would happen. 218. What would happen to the system if the pressure were decreased? A) Nothing would happen. B) More oxygen would be produced. C) The water vapor would become liquid water. D) The ammonia concentration would increase. E) The NO concentration would increase. 219. For a certain reaction at 25.0°C, the value of K is 1.2 10–3. At 50.0°C the value of K is 3.4 10–1. This means that the reaction is A) exothermic B) endothermic C) never favorable D) more information needed E) none of these (A-D) 220. Ammonia is prepared industrially by the reaction: N2(g) + 3H2(g) 2NH3(g) for the reaction: H° = –92.2 kJ and K (at 25°C) = 4.0 108. When the temperature of the reaction is increased to 500°C, which of the following is true? A) K for the reaction will be larger at 500°C than at 25°C. B) At equilibrium, more NH3 is present at 500°C than at 25°C. C) Product formation (at equilibrium) is not favored as the temperature is raised. D) The reaction of N2 with H2 to form ammonia is endothermic. E) None of the above is true. Use the following to answer questions 221-224. Consider the following equilibrium: 2H2(g) + X2(g) 2H2X(g) + energy 221. Addition of X2 to a system described by the above equilibrium A) will cause [H2] to decrease B) will cause [X2] to decrease C) will cause [H2X] to decrease D) will have no effect E) cannot possibly be carried out 222. Addition of argon to the above equilibrium A) will cause [H2] to decrease B) will cause [X2] to increase C) will cause [H2X] to increase D) will have no effect E) cannot possibly be carried out 223. Increasing the pressure by decreasing the volume will cause A) the reaction to occur to produce H2X B) the reaction to occur to produce H2 and X2 C) the reaction to occur to produce H2 but no more X2 D) no reaction to occur E) X2 to dissociate 224. Increasing the temperature will cause A) the reaction to occur to produce H2X B) the reaction to occur to produce H2 and X2 C) the reaction to occur to produce H2 but no more X2 D) no reaction to occur E) an explosion 225. Which of the following statements is true? A) When two opposing processes are proceeding at identical rates, the system is at equilibrium. B) Catalysts are an effective means of changing the position of an equilibrium. C) The concentration of the products equals that of reactants and is constant at equilibrium. D) An endothermic reaction shifts toward reactants when heat is added to the reaction. E) None of the above statements is true. 226. Consider the following system at equilibrium: N2(g) + 3H2(g) 2NH3(g) + 92.94 kJ Which of the following changes will shift the equilibrium to the right? I. increasing the temperature II. decreasing the temperature III. IV. V. VI. VII. VIII. increasing the volume decreasing the volume removing some NH3 adding some NH3 removing some N2 adding some N2 A) I, IV, VI, VII B) II, III, V, VIII C) I, VI, VIII D) I, III, V, VII E) II, IV, V, VIII 227. Consider the reaction A(g) + B(g) C(g) + D(g). You have the gases A, B, C, and D at equilibrium. Upon adding gas A, the value of K: A) increases, because by adding A more products are made, increasing the product to reactant ratio B) decreases, because A is a reactant so the product to reactant ratio decreases C) does not change, because A does not figure into the product to reactant ratio D) does not change, as long as the temperature is constant E) depends on whether the reaction is endothermic or exothermic 228. Consider the combustion of methane (as represented by the following equation). This is the reaction that occurs for a Bunsen burner, which is a source of heat for chemical reactions in the laboratory. CH4(g) + 2O2(g) CO2(g) + 2H2O(g) For the system at chemical equilibrium, which of the following explains what happens if the temperature is raised? A) The equilibrium position is shifted to the right and the value for K increases. B) The equilibrium position is shifted to the right and the value for K decreases. C) The equilibrium position is shifted to the left and the value for K decreases. D) The equilibrium position is shifted to the left and the value for K increases. E) The equilibrium position is shifted but the value for K stays constant. 229. Consider the reaction represented by the equation 2SO2(g) + O2(g) 2SO3(g). For the system at chemical equilibrium, which of the following explains what happens after the addition of oxygen gas (assume constant temperature)? A) The amount of SO3(g) increases and the value for K increases. B) The amount of SO3(g) decreases and the value for K increases. C) The amount of SO3(g) stays the same and the value for K decreases. D) The amount of SO3(g) decreases and the value for K stays the same. E) The amount of SO3(g) increases and the value for K stays the same. 230. Consider the reaction represented by the equation: N2(g) + 3H2(g) 2NH3(g). What happens to the equilibrium position when an inert gas is added to this system (as represented above) at equilibrium? A) If the container is rigid, nothing happens to the equilibrium position. If the container is fitted with a moveable piston, the equilibrium position shifts. B) If the container is rigid, the equilibrium position shifts. If the container is fitted with a moveable piston, nothing happens to the equilibrium position. C) The equilibrium position shifts no matter what the container is like. D) Nothing happens to the equilibrium position no matter what the container is like. E) The value of the equilibrium constant must be known to answer this question.