SCH 3UI Stoichiometry Practice Worksheet 2014
advertisement

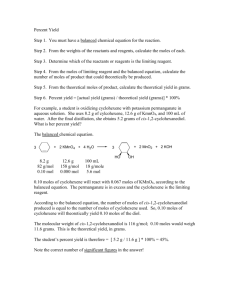
SCH 3UI Stoichiometry Practice Worksheet Name: ____________________________________________ Date: ____________________ A. General Stoichiometry Questions 1. 2. 3. List the 4 steps required in most general stoichiometry questions How can you identify a limiting/excess reagent question? How can you identify a percentage yield question? B. Mole Ratio Questions 1. 0.425 mol of Cu reacts with excess silver nitrate. a) How many moles of silver are produced? b) How many molecules of silver are produced? 2. In the following equation 2 C8H18(g) + 25 O2(g) → 16 CO2(g) + 18 H2O(g): a) If 1.00 mol of octane is burned , how many moles of carbon dioxide are produced? b) If 1.00 mol of octane is burned , how many moles of oxygen are required? c) If 12 mol of octane are burned , how many moles of water vapour are produced? d) If 9.0 mol of water vapour are produced, how many moles of octane are required e) If 4.36 mol of octane is burned , how many moles of carbon dioxide are produced? C. Standard Stoichiometry 1. In the production of ammonia, the following unbalanced reaction is used: ___ N2(g) + ___ H2(g) → ___ NH3(g) a) If 235.0 g of hydrogen gas are used, how much nitrogen gas is required? b) How much ammonia would be produced? 2. According to the balanced equation below: 2 Al(s) + 6 HCl(aq) → 2 AlCl3(aq) + 3 H2(g) a) Calculate the mass of hydrogen formed when 27.0 g of aluminum reacts with excess hydrochloric acid. b) If you were running this experiment, how would you know whether the reaction was complete? D. Limiting and Excess reagents 1. 138 g of NO2 is reacted with 54.0 g H2O to produce nitric acid, HNO3 according to the equation: 3 NO2 + H2O → 2 HNO3 + NO a) Which reactant is the limiting reagent? b) How much nitric acid is produced? c) What is the mass of the excess reagent? 2. Using the following equation: __ Pb(SO4)2 + __ LiNO3 __ Pb(NO3)4 + __ Li2SO4 a) If 20.0 g of lead (IV) sulfate reacts with 65.0 g of lithium nitrate, which reactant is limiting? b) How much lithium sulfate will be formed? E. Percentage Yield 1. In the direct reduction method of steelmaking, iron (III) oxide reacts with excess carbon monoxide to produce iron and carbon dioxide. 765 kg of iron ore (iron (III) oxide) reacts with excess carbon monoxide to produce 501 kg of pure iron. a) What is the percentage yield of this reaction? b) Chemical Engineers usually want the amount of excess reagent to be 10% more than required to make sure the reaction is complete. How much carbon monoxide would be required? 2. For the balanced equation: CaCN2 +3 H2O CaCO3 +2 NH3, if the reaction of 77.0 g of CaCN2 produces 27.1 g of NH3, what is the percent yield? F. Lab Question The following single displacement reaction is conducted: Al(s) + CuCl2(aq) → Cu + AlCl3(aq) in the lab (Note that the reaction is not balanced). Procedure: 1. A 25.0 g strip of Al is placed in a solution made from 5.00 g of CuCl2. 2. The excess reagent is removed. 3. When the reaction is complete the precipitate and the solution are poured through a filter. 4. The solution passes through the filter and the precipitate remains in the filter. 5. The filter is dried. Observations: 1. Mass of filter = 18.92 g 2. Mass of dried precipitate + filter = 20.96 g Analysis: 1. 2. 3. 4. 5. What is the limiting reagent? Looking at this reaction, how could you tell that it has gone to completion? What is the actual yield? What is the percentage yield? Suggest 3 potential errors that could happen in this experiment.