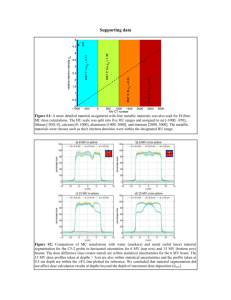
PET/CT Scanner Qualification Process: SNM Clinical Trials Network
advertisement
PET/CT Equipment Questionnaire Imaging Facility Name: Ship-To Address: City: State/Province : Country: Person performing phantom scans: Tel: Fax: Postal Code: Degree(s): Email: Person performing scanner QC: Email: Number of fixed PET/CT scanners available for ONCOLOGY Research: Provide information below - use additional sheets as necessary for more than three stationery PET/CT units A. SCANNER: Make/Model: #CT Slices: Year Manufactured: Crystal Type: Year Upgraded: Mode for Oncology: 2D Scanner Certification: DOSE CALIBRATOR: 3D None Other Capabilities: ACR Capintec Dose Calibrator Model: Type of Upgrade: ACRIN Atomlab B. SCANNER: Make/Model: 2D 3D None Dose Calibrator Model: ACRIN Atomlab ToF Cardiac Gating CQIE Veenstra THIS IS THE: #CT Slices: Year Manufactured: ICANL Respiratory Gating Other Other 2D 3D None original F-18 setting OR Type of Upgrade: Other Capabilities: ACR Capintec adjusted F-18 setting Crystal Type: Year Upgraded: Dose Calibrator Model: ACRIN Atomlab ToF Cardiac Gating CQIE Veenstra ICANL Respiratory Gating Other Other Year dose calibrator was installed: Current F-18 # on your dose calibrator: THIS IS THE: original F-18 setting OR Site able to: ~ transfer images electronically to a secure central server: ~ produce standard DICOM data sets: Yes No ~ employ a reconstruction matrix size of: 128 x 128 SOURCE FOR FDG adjusted F-18 setting Year dose calibrator was installed: C. SCANNER: Make/Model: DOSE CALIBRATOR: Other Type of Upgrade: ACR Current F-18 # on your dose calibrator: Scanner Certification: Other original F-18 setting OR Other Capabilities: Capintec Mode for Oncology: ICANL Respiratory Gating Crystal Type: Year Upgraded: DOSE CALIBRATOR: CQIE Veenstra THIS IS THE: #CT Slices: Year Manufactured: Scanner Certification: Cardiac Gating Year dose calibrator was installed: Current F-18 # on your dose calibrator: Mode for Oncology: ToF Yes Yes adjusted F-18 setting No // Burn CDs: No // 256 x 256 Yes Yes No No Name / Location: Regulatory Information: FDG provided under PERSON COMPLETING FORM Name: Email: NDA#: N or aNDA#: Date: Please fax or email this completed form to Jina Kim at jkim@snmmi.org / 703-667-5135 (fax) Version 7/28/15_oncology PET-CT Equipment Questionnaire