Chemistry Ch. 9.2
advertisement

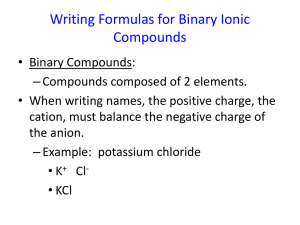
Chemistry Ch. 9.2 Naming and Writing Formulas for Ionic Compounds Naming Binary Ionic Compounds Binary compounds are composed of two elements and can be either ionic or molecular. If you know the formula for a binary ionic compound, you can write its name. Place the cation name first, followed by the anion name. NaBr is sodium bromide. SrF2 is strontium flouride. CuO is not just copper oxide. Copper can form 1+ and a 2+ cations. The formula indicates a 1 to 1 ratio. Since oxygen is always 2-, the correct name is copper(II) oxide. What is the formula for copper(I) oxide? Cu2O. Writing Formulas for Binary Ionic Compounds Easy. Write the symbol of the cation and then the anion. Add whatever subscripts are needed to balance the charges. Calcium bromide. Ca2+. Br-. CaBr2 Use the crisscross method, and then reduce to the lowest whole number ratio if necessary. Compounds with Polyatomic Ions Again easy. Write the symbol for the cation followed by the formula for the polyatomic ion, and then balance the charges. Use crisscross method. Ca2+ (NO3)Ca(NO3)2 Naming Compounds with Polyatomic Ions First, figure out if the compound contains a polyatomic ion! If so, then to name the compound, simply state the cation first and then the anion…the same as for binary ionic compounds. NaClO is sodium hypochlorite. What is (NH4)2C2O4 ?