280715120347SusChemE_2015_Abstract%5bBPSolanki
advertisement
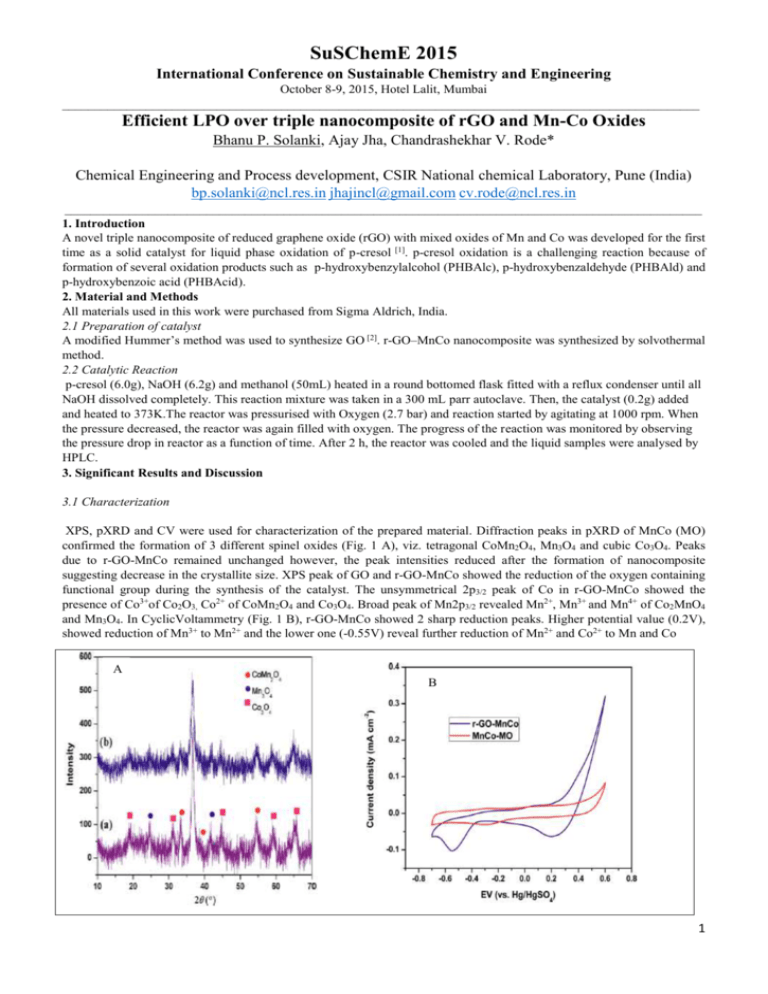
SuSChemE 2015 International Conference on Sustainable Chemistry and Engineering October 8-9, 2015, Hotel Lalit, Mumbai ___________________________________________________________________________________________________ Efficient LPO over triple nanocomposite of rGO and Mn-Co Oxides Bhanu P. Solanki, Ajay Jha, Chandrashekhar V. Rode* Chemical Engineering and Process development, CSIR National chemical Laboratory, Pune (India) bp.solanki@ncl.res.in jhajincl@gmail.com cv.rode@ncl.res.in ___________________________________________________________________________________________________ 1. Introduction A novel triple nanocomposite of reduced graphene oxide (rGO) with mixed oxides of Mn and Co was developed for the first time as a solid catalyst for liquid phase oxidation of p-cresol [1]. p-cresol oxidation is a challenging reaction because of formation of several oxidation products such as p-hydroxybenzylalcohol (PHBAlc), p-hydroxybenzaldehyde (PHBAld) and p-hydroxybenzoic acid (PHBAcid). 2. Material and Methods All materials used in this work were purchased from Sigma Aldrich, India. 2.1 Preparation of catalyst A modified Hummer’s method was used to synthesize GO [2]. r-GO–MnCo nanocomposite was synthesized by solvothermal method. 2.2 Catalytic Reaction p-cresol (6.0g), NaOH (6.2g) and methanol (50mL) heated in a round bottomed flask fitted with a reflux condenser until all NaOH dissolved completely. This reaction mixture was taken in a 300 mL parr autoclave. Then, the catalyst (0.2g) added and heated to 373K.The reactor was pressurised with Oxygen (2.7 bar) and reaction started by agitating at 1000 rpm. When the pressure decreased, the reactor was again filled with oxygen. The progress of the reaction was monitored by observing the pressure drop in reactor as a function of time. After 2 h, the reactor was cooled and the liquid samples were analysed by HPLC. 3. Significant Results and Discussion 3.1 Characterization XPS, pXRD and CV were used for characterization of the prepared material. Diffraction peaks in pXRD of MnCo (MO) confirmed the formation of 3 different spinel oxides (Fig. 1 A), viz. tetragonal CoMn2O4, Mn3O4 and cubic Co3O4. Peaks due to r-GO-MnCo remained unchanged however, the peak intensities reduced after the formation of nanocomposite suggesting decrease in the crystallite size. XPS peak of GO and r-GO-MnCo showed the reduction of the oxygen containing functional group during the synthesis of the catalyst. The unsymmetrical 2p3/2 peak of Co in r-GO-MnCo showed the presence of Co3+of Co2O3, Co2+ of CoMn2O4 and Co3O4. Broad peak of Mn2p3/2 revealed Mn2+, Mn3+ and Mn4+ of Co2MnO4 and Mn3O4. In CyclicVoltammetry (Fig. 1 B), r-GO-MnCo showed 2 sharp reduction peaks. Higher potential value (0.2V), showed reduction of Mn3+ to Mn2+ and the lower one (-0.55V) reveal further reduction of Mn2+ and Co2+ to Mn and Co A B 1 SuSChemE 2015 International Conference on Sustainable Chemistry and Engineering October 8-9, 2015, Hotel Lalit, Mumbai ___________________________________________________________________________________________________ Figure: 1. (A) XRD patterns and (B) CV curves of a) MnCo MO and b) r-GO–MnCo. 3.2 Catalyst activity Scheme 1. P-cresol oxidation Various catalysts were screened for p-cresol oxidation (Scheme 1) and the results are shown in Table1. It was reported that a minimum ratio of NaOH/p-cresol is required for oxidation reaction [3]. Among all the catalyst r-GO-MnCo (1:1) showed an excellent activity giving 71% yield of PHBAld with 96% selectivity. Table1: Catalyst screening for p-cresol oxidation Sr.No. Catalyst Yield Selectivity [%] [%] PHBAlc PHBAld PHBAcid 1 2 3 4 5 6 7 8 9 r-GO-Co r-GO-Mn MnCo(1:1) r-GO-MnCo(1:1) r-GO-MnCo(1:2) r-GO-MnCo(2:1) r-GO-MnCo(1:1) MnCo(1:1)/SiO2 MnCo(1:1)/Al2O3 20 28 42 71 57 60 NR[a] 9 31 100 1 1 PHBEth 89 10 1 95 96 97 95 3 3 2 3 1 1 1 1 93 93 6 6 1 1 [a] NR= No reaction(reaction performed without base) 4. Conclusion: Triple nanocomposite catalyst, r-GO-MnCo proved highly efficient for the selective liquid oxidation of p-cresol to PHBAld with a product yield of 71% and > 96% selectivity in 1 h. References [1] A. Jha, Sagar H. Patil, Bhanu P. Solanki , C.V. Rode, ChemPlusChem 2015,80, 1164-1169 [2] W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958,80, 1339. [3] C.V.Rode, M.V. Sonar, J.M. Nadgeri, Org. Process Res. Dev. 2004,8,873-878 2