AACI CRI Trial Complexity Form: Tool for Determining Institutional
advertisement

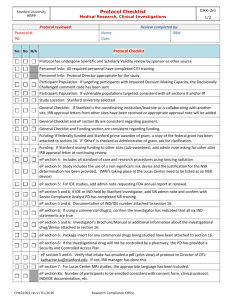
AACI CRI Trial Complexity Form: Tool for Determining Institutional Monitoring Standards TRIAL COMPLEXITY FACTORS I. FUNDING SOURCE Federal funding Externally Peer Reviewed Internally Funded (includes industry funded IIT) II. PHASE (Use current phase of trial and with regard to registration process) Phase 0 or pilot Phase I (Toxicity/dose finding) Phase II (Safety/Efficacy) Phase III (Effectiveness compared to recognized standard) Phase IV (post market trials) III. IND and/or IDE * IND/IDE (sponsor is your institution or PI) IND/IDE (sponsor is not your institution or PI) IND/IDE Exempt IV. US TRIAL SITES (External to your own site. Add 1pt for each site) External sites = __________ V. INTERNATIONAL TRIAL SITES (Add 1 pt. for each country) Country(s) = __________ VI. DRUG ADMINSTRATION PO or SQ (to be administered by the subject at home) IV or IM or SQ or other route to be administered by the clinical staff VII. MODALITY (add 1 pt. for ea. modality of treatment) # of modality(s) = ________ VIII. AGENT/ Device/Treatment/placebo Device Bone Marrow Transplant Gene transfer or viral vector Radio-labeled products Chemotherapy (oral) Chemotherapy (infusion) Stem cell transplant Biotherapy Standard radiation Nonstandard radiation IX. AGENT MANUFACTURING Cancer Center X. TISSUE OR BLOOD Simple or Single sample, (e.g., pre/post drug, pre-study, FU visit) Multiple and /or complex (e.g., liver biopsy, multiple timed PKs) Imaging Other (e.g. ultrasound guided biopsy) XI. ACCRUAL Trial accrual to be completed <12 months or site will enroll significant number of subjects, e.g., >10 XII. Investigative Research Personnel Experience PI experience 0 – 2 years Staff experience 0 – 1 year TOTAL Monitoring Levels Low Moderate High Risk SCORING OF RISK Total Ranges 0 to 15 Points 16 to 29 Points > 30 Points FORM INSTRUCTIONS: 1.) Assess Trial Complexity Factors (I – XII) completing the Trial Complexity Form (TCF) for each selected interventional investigator initiated trial. 2.) Each complexity factor is assigned a value 1-3 with a score of 3 being the highest. *IND or IDE institutionally held studies receive an automatic value of 10. This is the only Complexity Factor exceeding the 1-3 scoring AACI CRI Trial Complexity Form: Tool for Determining Institutional Monitoring Standards FORM INSTRUCTIONS: 1.) Assess Trial Complexity Factors (I – XII) completing the Trial Complexity Form (TCF) for each selected interventional investigator initiated trial. 2.) Each complexity factor is assigned a value 1-3 with a score of 3 being the highest. *IND or IDE institutionally held studies receive an automatic value of 10. This is the only Complexity Factor exceeding the 1-3 scoring