Protocol - Medical - Human Subjects
advertisement

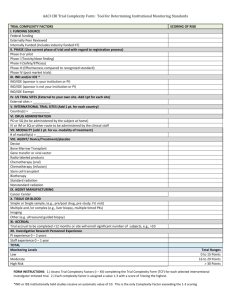
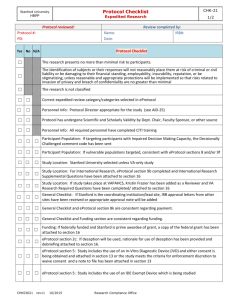
Stanford University HRPP Protocol Checklist CHK-2m Medical Research, Clinical Investigations 1/2 Protocol reviewed: Protocol #: PD: Review completed by: IRB#: Name: Date: Protocol Checklist Yes No N/A Protocol has undergone Scientific and Scholarly Validity review by sponsor or other source Personnel Info: All required personnel have completed CITI training Personnel Info: Protocol Director appropriate for the study Participant Population: If targeting participants with Impaired Decision Making Capacity, the Decisionally Challenged comment code has been sent Participant Population: If vulnerable populations targeted, consistent with eP sections 8 and/or 9f Study Location: Stanford University selected General Checklist: If Stanford is the coordinating institution/lead site or is collaborating with another site, IRB approval letters from other sites have been received or appropriate approval note will be added General Checklist and eP section 8k are consistent regarding payment. General Checklist and Funding section are consistent regarding funding. Funding: If federally funded and Stanford prime awardee of grant, a copy of the federal grant has been attached to section 16. If ‘Other’ is checked as Administrator of grant, ask for clarification. Funding: If Stanford issuing funding to other sites (sub-awardees), add admin note asking for other sites IRB approval letter at continuing review. eP section 4: Includes all standard of care and research procedures using ionizing radiation eP section 5: Study includes the use of a non-significant risk device and the justification for the NSR determination has been provided. (MRI’s taking place at the Lucas Center need to be listed as an NSR device) eP section 5: For IDE studies, add admin note requesting FDA annual report at renewal. eP section 5 and 6, if IDE or IND held by Stanford Investigator, add SIR admin note and confirm with Senior Compliance Analyst PD has completed SIR training eP section 5 and 6: Documentation of IND/IDE number attached to section 16. eP section 6: If using a commercial drug(s), confirm the Investigator has indicated that all six IND statements are true eP section 5 and 6: Investigator’s Brochure/Manual or additional information about the investigational drug/device attached to section 16. eP section 6: Package insert for any commercial drugs being studied have been attached to section 16. eP section 6: If the Investigational drug will not be controlled by a pharmacy, the PD has provided a Security and Controlled Access Plan eP section 5 and 6: Verify that intake has emailed a pdf (print view) of protocol to Director of OTL katharine.ku@stanford.edu. If not, IRB manager has done this. eP section 7: For Lucas Center MRI studies, the appropriate language has been included. eP section 8a: Number of participants to be enrolled consistent with consent form, clinical protocol, IND/IDE documentation, etc. CHK01002 rev11 01/2016 Research Compliance Office Stanford University HRPP Protocol Checklist CHK-2m Medical Research, Clinical Investigations 2/2 Protocol Checklist Yes No N/A eP section 8g: The patient will initially be informed about the study by someone they have a treating relationship with or the patient’s physician has obtained approval from the patient to be contacted by study team eP section 9a, if high risk protocol, consult with senior management regarding whether an expert review is required and notify Senior Management by email about review of the high risk protocol. eP section 9e, Data Safety Monitoring Plan included for all protocols that are greater than minimal risk. Add admin note has been added regarding frequency of DSMB/DMC reports if specified eP section 9f: The appropriate child risk determination has been made (FDA vs. OHRP, more than one child risk determination, etc.) and justification provided eP section 9f: For research including pregnant women or fetus, the required conditions have been met. eP section 11b: includes both health information and identifiers and is consistent with eP section 15 and the HIPAA Authorization section in the consent form. eP section 12: OPACS completed for all listed Investigators eP section 12: If COI indicated, action report has been received and attached to section 16 and appropriate language added to consent form. eP section 13 and 15: Short form consent process and alteration of HIPAA Authorization requested eP section 13 and 15: Waiver of documentation for phone screening and waiver of HIPAA Authorization for recruitment requested . eP section 13: Phone screen does not include any sensitive information (e.g. psychiatric diagnoses, symptoms of depression, illegal drug use) eP section 14: If the study includes children age 7 to 17, assent form, waiver of assent, or n/a has been provided or indicated. eP section 14: For children who become adults during active participation, details regarding the plan to re-consent the child as an adult has been provided eP section 15: If PHI will be obtained prior to obtaining consent, waiver of HIPAA Authorization for recruitment has been requested and only minimum necessary information to determine eligibility will be collected. eP section 16: Questionnaires attached to section 16 eP section 16: Advertisements/recruitment materials attached to section 16 eP section 16: All additional checklists (i.e. VA, DoD, ED, DOE, DOJ, EPA, etc.) have been completed CHK01002 rev11 01/2016 Research Compliance Office





![Assumptions Checklist [Word File]](http://s3.studylib.net/store/data/005860099_1-a66c5f4eb05ac40681dda51762a69619-300x300.png)