Acid-Base Webquest
advertisement

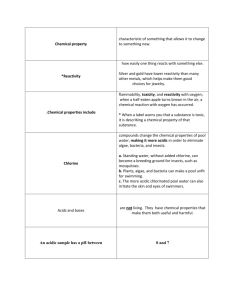
Mr. Shepherd’s Physical Science Name:________________________________ Acid-Base Webquest If you have not already done so, you should print off the Acid-Base Webquest Questions that are on a link in the Webquest section of this website. Some of the questions you may be able to answer without checking the websites if you remember concepts that we covered in class. There are links below that you can visit to find information related to acids and bases. Use this link to answer the following questions: http://www.files.chem.vt.edu/RVGS/ACT/notes/Notes_on_acids_and_bases.html Resources General Acid-Base Information Acid-Base Tutorial & Problems Properties of Acids & Bases pH Information Strong vs. Weak Acids Strong vs. Weak Bases More pH Info w/ animations pH Animation Acid Base Indicators#1 Acid Base Indicators#2 Searchable Acid Base FAQ database http://www.chemtutor.com/acid.htm http://www.biology.arizona.edu/biochemistry/problem_sets/ph/ph.html http://virtual.yosemite.cc.ca.us/lmaki/Chem142/chap_outlines/chapter12.htm http://www.purchon.com/chemistry/ph.htm http://www.chemguide.co.uk/physical/acidbaseeqia/acids.html http://www.chemguide.co.uk/physical/acidbaseeqia/bases.html http://web.jjay.cuny.edu/~acarpi/NSC/7-ph.htm http://www.johnkyrk.com/pH.html http://chemistry.about.com/od/acidsbases/a/Acid-Base-Indicators.htm http://www.chemguide.co.uk/physical/acidbaseeqia/indicators.html http://antoine.frostburg.edu/chem/senese/101/acidbase/faq.shtml Mr. Shepherd’s Physical Science Name:________________________________ 1.) Describe the physical properties, taste, feel, etc, of Acids. And of Bases. Acids: _________________________________________________________________________________________ Bases: ____________________________________________________________________________________ 2.) Give 3 examples of acids and 3 examples of bases from the pH Scale on this document: Acids: 1. 2. 3. Bases: 1. 2. 3. 3.) List the metals that acids react with to form H2 gas:___________________________________ 4.) When acids react with react with compounds containing CO32- what products are formed?________________________________________________ 5.) Acids turn litmus ________________ . 6.) Bases turn litmus _______________ . 7.) Bases react with most cations to precipitate _________________________. 8.) What is the difference between an electrolyte and a nonelectrolyte? __________ ________________________________________________________________ 9.) How can pH be determined experimentally? ________________________________________ . 10.) How do you neutralize an acid? _______________________________________ A base? _________________________________________________________ 11.) Watch the pH animation and describe the role of an acid and of a base in an acid/base reaction. 12.) How is a strong acid different from a weak acid? 13.) How is a strong base different from a weak base? 14.) List the six strong acids: ___________________________________________________________ . 15.) Why do we use a double arrow in( ↔ ) the dissociation equation for a weak acid? _________________________________________________________________________________ . 16.) Why is only one arrow ( → ) required for the dissociation of a strong acid? _____________________________________________________________________ . 17.) List the 3 scenarios for a substance being classified as a weak acid: a.) _______________________________________________________________ . b.)________________________________________________________________ . c.) _______________________________________________________________ . Mr. Shepherd’s Physical Science Name:________________________________ 18.) Describe strong bases: ______________________________________________ . 19.) Which are the strong bases?__________________________________________________ . 20.) How many moles of OH ions are produced from the hydroxides of Group II metals for every mole of base that dissociates? _________________ . 21.) Give the general equation for the reaction of a weak base with water: 22.) What do scientists use to measure pH? ________________________________ 23.) pH is measured on a logarithmic scale. What does this mean? ________________________________________________________________ ________________________________________________________________ 24a. What is the difference between pH and pOH? (equation) 25b. What do the brackets around a chemical mean, like for [H+]? ____________________________________________________________________ Use this link to answer the following questions: http://www.wwnorton.com/college/chemistry/gilbert2/tutorials/interface.asp?chapter=chapter_16 &folder=acid_rain 26.) Rain that has a very low pH is called ____________________________ . 27.) Natural rainwater has a pH of ______________ . 28.) Sulfur dioxide reacts with atmospheric oxygen to form ______________ . 29.) Name the two acids in pollution that make rain acidic: _____________________ . 30.) Fish in a lake will die if the pH drops below _____________ . Use this link to answer the following questions: http://www.wwnorton.com/college/chemistry/gilbert2/tutorials/interface.asp?chapter=chapter_16 &folder=strong_titrations 31.) A titration is the ___________________________________________________ . 32.) The solution of known concentration is called the _________________________ . 33.) The initial volume is read from the bottom of the _________________________ . 34.) The equivalence point of a titration is when______________________________ . 35.) The course of the reaction during a titration can be followed by plotting the pH on a _________________________________ . 36.) The pH of the solution at the equivalence point is determined by ________________________________________ . 37.) What is an indicator? ______________________________________________ . 38.) What color is methyl red at pH = 8? ______________ . 39.) In acidic solutions ________________________ is colorless. 40.) When base is added, phenolphthalein will remain colorless until the pH = _____ . 41.) At the equivalence point of the titration, the color of the solution will be __________ . Mr. Shepherd’s Physical Science Name:________________________________