Topic 8 * Reaction Rates
advertisement

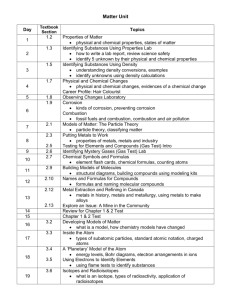
Topic 8 – Reaction Rates Reaction Rate • A _______________________________ is a measure of how fast a reaction occurs – To measure: • Find out how quickly one of the reactants is disappearing OR • Find out how quickly one of the products is appearing. Can the Reaction Rate be changed? • 1. _____________________________________________________ • 2. _____________________________________________________ • 3. Adding a Catalyst • 4. Inhibitors • 5. ______________________________________________________ Inhibitors • _________________________ are substances that ______________________________ down chemical reactions. – Inhibitors are added to some foods and medicines to slow down their decomposition. Catalysts • A ____________________ is a substance that _________________________ up the rate of a reaction without being changed itself. Enzymes • An ____________________________________ is a NATURAL catalyst made by living things. – Example: • When you chew up that delicious sandwich, glands in your mouth produce saliva which contains _________________________________ to break down food. – The enzyme in saliva works as a catalyst to break down food. Temperature • The rate of a chemical reaction can be sped up or slowed down by changing the _________________________________________ – At _____________________________ temperatures, reactions will increase in speed – At _____________________________ temperatures, reactions will decrease in speed Stirring • _______________________________________ a mixture will usually make a reaction happen more quickly – Think about salt dissolving in water, if you stir it, the salt will dissolve MUCH quicker Corrosion • When metals are exposed to air and moisture, what happens??? – ______________________________________ • _____________________________ is the oxidation of metals or rocks in the presence of air and moisture – __________________________ is an example of corrosion (rust is called iron (II) oxide trihydrate) • Rust -> Iron + Oxygen + Moisture Poor Metals…. • Corroded materials lose their strength Pooooor metals…. – Things like rust will eventually make its way through the metal and it will all corrode away…. • • NO more metals…… HOW DO WE SAVE THE METALS FROM THEIR DEMISE?!?! Preventing Corrosion • 1. Apply a thin coat of paint. • 2. Coat the metal with zinc (way more resistant to corrosion) • Coating metals with a thin layer of ___________________________ is called ___________________________________________ – Who can think of some metals that are galvanized?