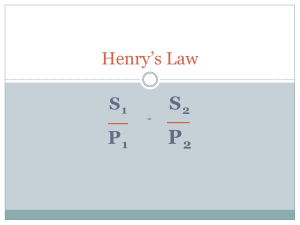
Henry`s Law Practice

Henry’s Law Practice Problems
How many grams of oxygen (O
2
) can be dissolved in 1 L of water at 20
C if the oxygen pressure is 2.00 atm. The
Henry's Law constant (k) for oxygen at 20
C is 1.38 x 10
-3
M/atm.
If 0.55 g of a gas dissolves in 1.0 L of water at 2 atm of pressure, how much will dissolve at 4.5 atm of pressure?
(1.23 g/L)
The solubility of methane, the chief component of Bunsen burner gas, in water at 20
C and 1.00 atm of pressure is
0.025 g/L. What will be its solubility at 1.4 atm and 20
C?
At 98366 kPa and 20
C nitrogen has a solubility in water of 0.018g/L. At 82.66 kPa and 20
C it’s solubility is 0.015 g/L. Does nitrogen obey the gas pressure-solubility law?
A gas has a solubility of 0.66 g/L at 10 atm of pressure. What is the pressure on a 1.0 L sample that contains 1.5 g of gas? (22.73 atm)
If 0.68 g of a gas at 5.00 atm of pressure dissolves in 1.0 L of water at 25 o
C, how much will dissolve in 1.0 L of water at 8.00 atm of pressure and the same temperature? (1.09 g/L)
A gas has a solubility of 1.46 g/L at 8.00 atm of pressure. What is the pressure of a 1.0 L sample that contains 2.7 g/L? (14.8 atm)
If 1.2 g of a gas at 6.00 atm of pressure dissolves in 1.0 L of water at 25 o C, how much will dissolve in 1.0 L of water at 3.00 atm of pressure and the same temperature?
If 0.24 g of gas dissolves at 1.0 L of water at 1.5 atm of pressure, how much gas will dissolve if the pressure raises to
6.0 atm.
A gas has a solubility of 0.086 g/L at a pressure of 3.5 atm. At what pressure would its solubility be at 2.3 g/L?
The solubility of a gas changes form 0.95 g/L to 0.72 g/L. If the initial pressure was 2.8 atm, what is the final pressure?