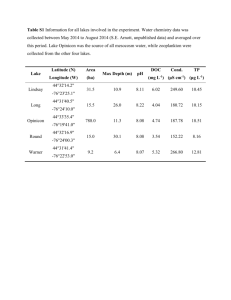
Supplementary Materials Figure S1. Growth and contour
advertisement

Supplementary Materials Figure S1. Growth and contour-stepped differential response surfaces for descendent (blue) and ancestor (yellow) strains across a carbon and nitrogen gradient. The descendent strains, Low-N (a-b) and Low-C (c-d), were predicted to be competitively dominant over the ancestral strain everywhere except for low nitrogen concentrations, driven by increased RK values and increased affinity for both carbon and nitrogen. METHODS Saccharomyces cerevisiae Isolates The strains were isolated from a “no captive cultures” winery (Kumeu River, Auckland, New Zealand). For the work presented here, we used two wild-type, diploid isolates with differing genotypes based on micro-satellite typing of five loci; each isolate being <50 generations removed from the wild. These isolates are referred to as P1 and P2 following the original identification by Goddard (2008). The isogenic strains were stored at −80 °C with 1.7 mL of dense culture and 0.3 mL glycerol (15% v/v) in 2 mL Eppendorf tubes; this is standard practice for storage of S. cerevisiae cultures. For the purposes of our work we considered an isogenic strain a population, which enabled unambiguous identification of niche requirements, competitive outcomes and evolutionary responses. Specifically, use of isogenic strains removes within-population variation associated with the presence of different genotypes (polygenic populations). Isolates were sub-sampled from cryo-storage into standard media for culturing S. cerevisiae. They were inoculated into yeast extract-peptone-dextrose broth (YPD; Y1375, Sigma-Aldrich, St. Louis, MO, USA), which at 50 g L-1 comprises 20 g L-1 bacteriological grade peptone, 10 g L-1 yeast extract, and 20 g L-1 glucose. Chemostat Cultures Experiments were performed in Multitron II incubators (Infors HT, Bottmingen, Switzerland) with DasGIP glassware (DasGIP AG, Jülich, Germany) and “200 Series” pumps (Watson- Marlow, Wilmington, MA, USA). Each of these chemostats concurrently maintained sixteen 250-mL culture flasks at 30°C. Chemostat culture flasks were inoculated from starter cultures of Saccharomyces cerevisiae and grown at 30°C for 48 h in batch-culture mode with non-limiting nutrients, after which they were inoculated into the chemostats and maintained under log-phase growth with constant resource-supply rates. Initial tests with different incubation times suggested that populations had stable biomass values after ≥25 generations; we therefore mapped population niche requirements after 25 generations growth in the chemostat. This number of generations also limited the possibility that a beneficial mutation might sweep through any one population (Goddard & Bradford 2003). Initial tests with different carbon (C) and nitrogen (N) supply rates suggested that concentrations of C ranging from 0.09 to 0.75 g C L-1 gave ~10-fold differences in population biomass when N was not limiting (0.064 g N L-1), and that concentrations of N ranging from 0.004 to 0.032 g N L1 gave ~12-fold differences in population biomass when C was not limiting (1.5 g C L-1). The medium (CYN5501 yeast nitrogen base without amino acids, (NH4)3PO4, KH2PO4 and Inositol; ForMedium Ltd, Norwich, UK) for overnight cultures at non-limiting nutrient conditions was combined with 10 g L-1 D-glucose monohydrate (49159; Sigma-Aldrich, St. Louis, MO, USA), 5 g L-1 anhydrous (NH4)2SO4 (A4418; Sigma-Aldrich, St. Louis, MO, USA), and 1 g L-1 anhydrous KH2PO4 (P9791; Sigma-Aldrich, St. Louis, MO, USA). This gave concentrations of 4.00 g L-1 carbon, 1.06 g L-1 nitrogen, and 0.228 g L-1 phosphorous. Each medium was adjusted to pH 6.0±0.1 using NaOH. We established in preliminary experiments and development of our ‘Characterization Protocol’ that culture densities in the chemostat with this medium was equivalent at the stated and double concentrations of C, N and P, confirming the formulation was not growth-limiting. To invoke resource limitation we then fed the chemostat cultures with the same base medium but with reduced concentrations of C and N. The reference, “rich” formulation of our experimental culture medium provided 3.20 g C L-1, 1.00 g N L-1 and 0.200 g P L-1; as well as the manufacturer’s recommended concentration of YNB without amino acids. The P and base concentrations were held at these concentrations for the entire process (including under the evolution experiments). The C supply concentration of 3.20 g L-1 was below the 8 g L-1 threshold considered to drive experimental populations into fermentative, anaerobic respiration. Biomass was determined by ceasing the chemostat agitation and allowing the cultures to stand for 5 min to permit the non-cellular residue to settle to the base of the flasks, at which point ~45 mL of culture was removed. The sample’s exact volume was determined before it was centrifuged at 4 °C to collect the biomass pellet. The supernatant was then removed and the pellet re-suspended by vortex agitation in 1 mL DI water and transferred to a 2 mL microcentrifuge tube. The process of centrifugation and re-suspending was repeated twice more to wash the biomass clean of media. The cleaned pellet was dried at 65 °C to stable mass (~24 h), and then weighed. From the mass and volume of culture sampled we estimated the population mass density to be used in the second step of the characterization protocol. The two chemostats were maintained with gyratory agitation at 105 rpm and 25 mm throw, 10.7 mL h-1 feed rate, 50±2 mL working culture volumes, and 4.69 h (0.213 h-1) culture turnover times. Each flask had a separate nutrient medium supply, waste removal, and air supply. Waste removal was siphoned at 3 times the rate of media supply, ensuring that culture volumes were maintained at ~50 mL and that there was no back-flow of waste. Air supply (at 0.5 L min-1) was passed through oil and water traps and filtered to 0.2 µm. The outlet filter on each flask maintained a positive pressure within each flask, in an attempt to prevent microbial contamination of the cultures. The number of generations in our ‘Characterization cultures’ was below that required for a mutation to sweep to high frequency in the experimental populations. Given estimates of the rate and average fitness effects of beneficial mutation in yeast (Joseph and Hall 2004), multiple beneficial mutations would arise each generation and escape Haldane’s sieve (Haldane 1927), since population sizes are ~108 cells. However, the initial frequency of a newly arising mutation will be small, equal to the reciprocal of the population size, i.e. ~10-8, implying that it would take numerous generations for a beneficial allele to become common in the culture population (Haldane 1924). We thus do not expect any beneficial mutations to go to high enough frequency during the 25 generations of characterization growth to alter the resource utilization parameters of the population. Previous work corroborates this expectation; only a handful of beneficial mutations were identified following two thousand generations of selection in diploid yeast (Zeyl 2005). Antibiotic Resistance Antibiotic-resistant strains were constructed using the pYMN21 nourseothricin resistance (NATR) plasmid following standard protocols (Janke et al. 2004). The goal was to insert the NATr gene at position 49550 of chromosome IV. In other strains, this insertion does not affect fitness (DWH, personal observation). The pYMN21 plasmid was obtained from Euroscarf (Institute for Molecular Biosciences, Frankfurt, Germany). Briefly, primers were designed to amplify the NATR gene from the plasmid. The upstream primer had 50 bases identical to positions 49501-49550 of chromosome IV followed by19 bases of identity to the plasmid region S1 (Janke et al 2004). The downstream primer had 50 bases identical to positions 49600-49551 of chromosome IV followed by 20 bases of identity to the plasmid region S4 (Janke et al 2004). Sequences of the primers are as follows. Bases in bold at 3’ end of primers are identical to plasmid sequences, bases in regular typeface are identical to appropriate regions of chromosome IV. Upstream primer: 5’GCTCCGCAACTTGCTTGAATTATGAGCTCTAAGATTCAAGAAGTAAATAGCGTACGC TGCAGGTCGAC3’ Downstream primer: 5’TAAGATCACATGGTTCCTTTATCAAGTACTACTATCATTCCATTATATGACATCGATG AATTCTCTGTCG3’ Resistant transformants were selected on nourseothricin plates (100 µg L-1), and insert location confirmed by PCR. We also confirmed that the antibiotic-resistant isolates showed robust colony growth on both plate types, and that our antibiotic-naïve isolates only grew on the standard plates. The naïve strains retained the designations P1 and P2, and the resistant strains were named P1R and P2R. Determining Competitive Outcomes To perform a competition, the two selected strains were grown separately in standard YPD medium (described above) to between 1×107 and 5×107 cells mL-1. Each culture was adjusted (diluted) with standard YPD medium to 5×106 cells mL-1 and 100 mL aliquots of the adjusted cultures were mixed. The resulting mixed culture was used to inoculate the chemostat flasks for the competition run: 10 mL of combined culture was added to each chemostat flask containing 50 mL of competition medium. This gave starting densities of ~4.2×106 cells mL-1 of each strain. The competition medium was the chemostat base medium (described above) with concentrations of C and N defined by the resource supply point being investigated. After 25 generations, media flow and agitation were stopped and the chemostat flasks were allowed to stand for 5 min to permit the non-cellular residue to settle. Next ~35 mL of culture was removed and population density measured using a T100 Turbidity Meter (Oakton Instruments, Vernon Hills, IL, USA). Then each mixed culture was diluted to ~2×104 cells mL-1 and 10 µl (~200 cells) of this dilution was inoculated onto four, 10-cm dia. agar plates and distributed evenly by spreading with three, 5-mm diameter glass balls. Adaptation to Nutrient Limitation We investigated the ability of our model to identify how traits and competitive abilities differ among genetically related individuals. To do this, we selected P1 as an ancestral population and adapted it to two separate resource-limited regimes. The “Low-C” medium provided 0.0550 g C L-1 and 0.0470 g N L-1, a C:N ratio of 1.17. The “Low-N” medium provided 1.80 g C L-1 and 0.00290 g N L-1, a C:N ratio of 621. The dual-limiting or “optimum” C:N ratio for the P1 isolate (see Results), was 16.1. The C:N ratio in the “Low-N” environment was thus 38.5 times the optimum and in the “Low-C” environment it was 0.076 times optimum. Phosphorus and YNB w/o amino acids concentrations were held at constant, non-limiting levels. We ran sixteen chemostat adaptation cultures – eight “Low-N” (i.e., ‘high C:N’) and eight “Low-C” (i.e., ‘LowC:N’) – for 79 days (404 generations at 4.69 h turnover). Culture samples were aseptically extracted at several checkpoints and cryo-stored. We checked the density of each terminal culture and selected the one from each medium that exhibited the highest density for characterization and comparison with our growth response model. These were designated as “Low-N” and “Low-C” isolates. Table S1. The eight carbon (C) and nitrogen (N) concentrations under which growth rates were measured for each fungal population and used to estimate growth trait parameters and corresponding niche spaces. C, g L-1 N, g L-1 0.094 0.188 0.064 0.375 0.750 1.500 0.032 0.016 0.008 0.004 Table S2. Initial round of competitive pairings with 8 replicates each and an exact enumeration of CFU counts. [C]: carbon resource concentration, g L-1; [N]: nitrogen resource concentration, g L-1; Pop. Y: non-antibiotic-resistant population; Pop. Z: antibioticresistant population; RY: predicted realized growth rate of population Y; RZ: predicted realized growth rate of population Z; ΔR: difference of predicted realized growth rates (RZ-RY); Prop. Z: mean proportion of antibiotic-resistant CFUs across the 8 replicates (calculated as ‘CFUs on antibiotic plate / Total CFUs’); S.E.: standard error of the proportion; 95% C.I.: 95% confidence interval of the proportion; P: p-value testing the null hypothesis that the proportion is equal to 0.5; Pred. Win: predicted winner based on ΔR; Win by Prop: winning strain based on relative proportion of CFUs on antibiotic plates relative to standard plates; Win by CI: winner based on confidence interval being less than, greater than, or crossing 0.5. The symbol ‘?’ indicates stochastic dominance (Predicted Winner column) or that no clear winner was observed across the replicates (Actual Winner). Actual Winner 0.069 0.64 (0.52, 0.73) 0.025 P2R P2R 0.709 -0.051 0.40 (0.35, 0.46) 0.004 P2 P2 0.642 0.641 -0.001 0.43 (0.31, 0.54) 0.179 ? ? 0.370 0.372 0.002 0.48 (0.31, 0.64) 0.756 ? ? 0.710 0.778 P2 P1R 0.759 P1 P1 R P2 R R P1 1.2 0.08 0.01 Predicted Winner P2R 0.08 0.2 P ΔR 1.2 0.08 95% C.I. RZ [N] 0.7 Prop. Z RY [C] Pop. Y Pop.Z P2 0.3 0.04 Low-N P1 0.542 0.485 -0.057 0.09 (0.00, 0.24) <0.001 Low-N Low-N 0.6 0.02 Low-N P1R 0.534 0.478 -0.056 0.26 (0.18, 0.33) <0.001 Low-N Low-N Low-N P1 R 0.690 0.641 -0.049 0.16 (0.00, 0.35) 0.005 Low-N Low-N P1 R 0.842 0.814 -0.028 0.21 (0.17, 0.26) <0.001 Low-N Low-N R 0.7 6.0 0.05 0.40 Low-N 0.3 0.04 Low-C P1 0.540 0.485 -0.055 0.30 (0.25, 0.34) <0.001 Low-C Low-C 0.3 0.4 Low-C P1R 0.540 0.485 -0.055 0.30 (0.21, 0.39) 0.002 Low-C Low-C Low-C P1 R 0.604 0.559 -0.045 0.27 (0.21, 0.34) <0.001 Low-C Low-C P1 R 0.840 0.811 -0.029 0.25 (0.16, 0.33) <0.001 Low-C Low-C 0.6 5.6 0.03 0.36 Low-C Table S3. Second round of competitive pairings with 2 replicates each and a visual estimation of CFU proportions. [C]: carbon resource concentration, g L-1; [N]: nitrogen resource concentration, g L-1; Pop. Y: non-antibiotic-resistant population; RY: predicted realized growth rate of population Y; Pop. Z: antibiotic-resistant population; RZ: predicted realized growth rate of population Z; ΔR: difference of predicted realized growth rates (RZ-RY); Pred. Win: predicted winner based on ΔR; Win by Prop.: winning strain based on relative proportion of CFUs on antibiotic plates relative to standard plates. The symbol ‘?’ indicates that either stochastic dominance was predicted or that no clear winner was observed across the replicates. [C] [N] Pop. Y Pop. Z RY RZ ΔR Pred. Win Win by Prop. 0.2 0.010 P1 P1R 0.363 0.333 -0.030 P1 P1 P1 P1 R 0.363 0.333 -0.030 P1 P1 P1 R 0.407 0.400 -0.007 ? ? P1 R 0.710 0.713 0.003 ? R 0.491 0.524 1.2 0.2 1.2 0.010 0.100 0.100 P1 P1 ? 0.033 P2 R P2R 0.3 0.025 P1 P2 1.2 0.025 P1 P2R 0.549 0.581 0.032 P2R P2R 0.3 0.100 P1 P2R 0.491 0.524 0.033 P2R P2R 1.2 0.100 P1 P2R 0.710 0.778 0.069 P2R P2R 0.3 0.025 P2 P1R 0.521 0.485 -0.036 P2 P2 R 1.2 0.025 P2 P1 0.571 0.523 -0.049 P2 P2 0.3 0.100 P2 P1R 0.521 0.485 -0.036 P2 P2 1.2 0.100 P2 P1R 0.759 0.713 -0.047 P2 P2 P2 P2 R 0.370 0.372 0.001 ? P2 P2 R 0.370 0.372 0.001 ? ? R 0.431 0.430 -0.001 ? P2 0.759 0.778 0.019 P2R P2R 0.2 1.2 0.010 0.010 P2 0.2 0.100 P2 P2 1.2 0.100 P2 P2R References 1. Goddard, M.R. & Bradford, M.A. (2003). The adaptive response of a natural microbial population to carbon- and nitrogen-limitation. Ecol. Lett., 6, 594–598. 2. Janke, C., Magiera, M.M., Rathfelder, N., Taxis, C., Reber, S., Maekawa, H., et al. (2004). A versatile toolbox for PCR-based tagging of yeast genes: New fluorescent proteins, more markers and promoter substitution cassettes. Yeast, 21, 947–962.